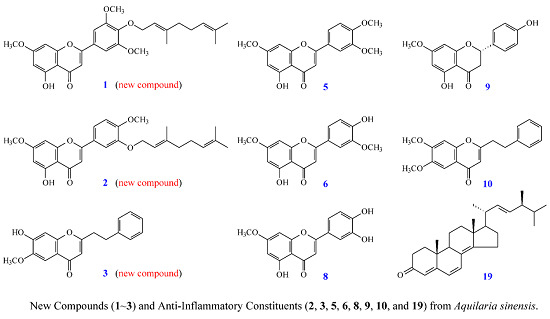
New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis
Abstract
:1. Introduction
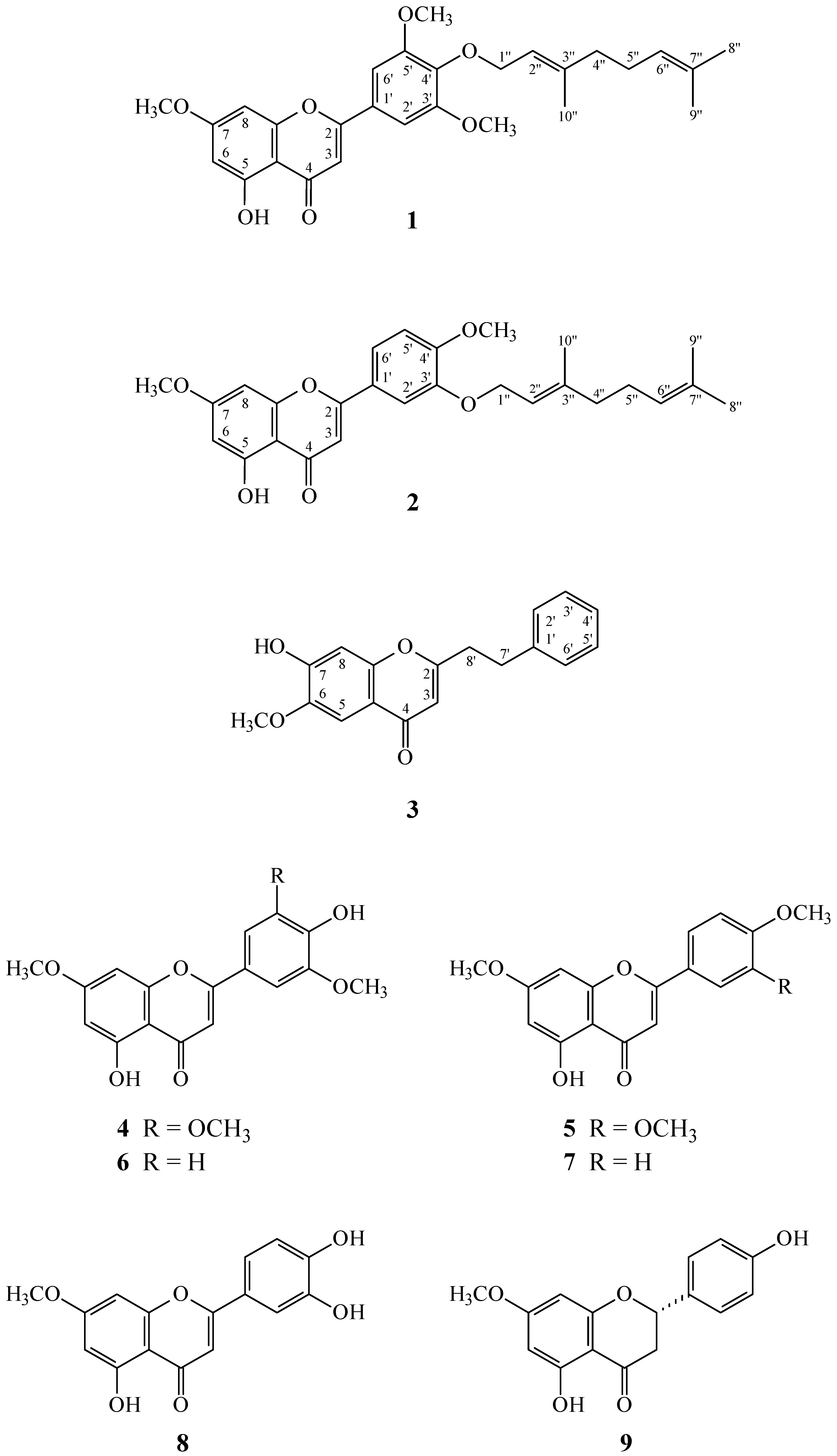

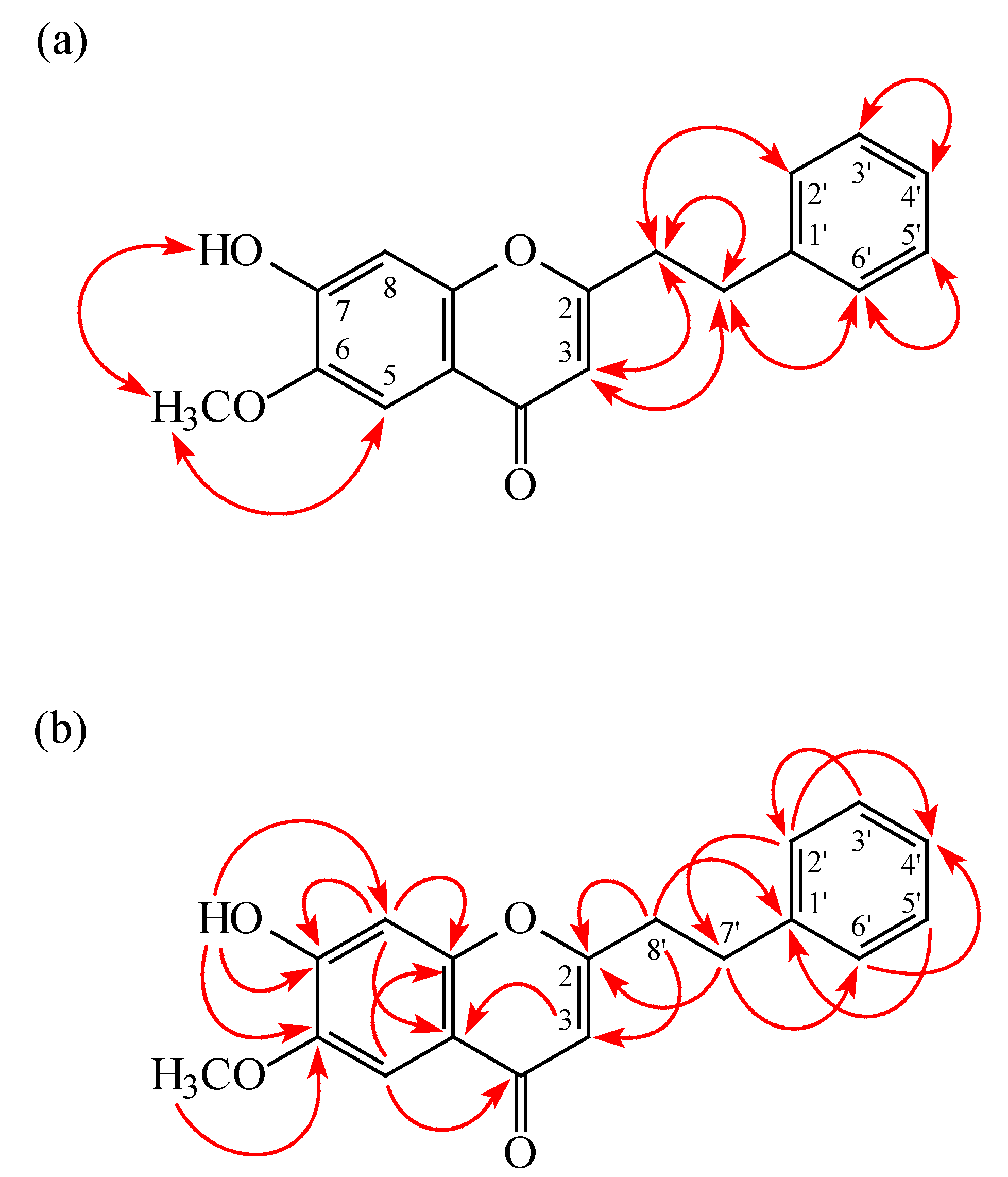
2. Results


| Compound | IC50 (μM) a or (Inh %) b | |
|---|---|---|
| Superoxide Anion Generation | Elastase Release | |
| 4′-O-Geranyltricin (1) | (13.23 ± 6.82) | (12.80 ± 6.84) |
| 3′-O-Geranylpolloin (2) | 12.51 ± 2.75 e | (17.34 ± 3.81) c |
| 7-Hydroxy-6-methoxy-2-(2-phenylethyl)chromone (3) | 4.62 ± 1.48 e | 3.91 ± 0.87 e |
| Tricin (4) | (3.61 ± 2.29) | (17.69 ± 1.71) e |
| 5-Hydroxy-7,3′,4′-trimethoxyflavone (5) | 4.69 ± 0.94 e | (9.32 ± 1.37) e |
| Velutin (6) | 1.78 ± 0.35 e | 4.26 ± 0.12 d |
| Apigenin 7,4′-dimethyl ether (7) | elicit superoxide anion generation and elastase release | |
| 3′-Hydroxygenkwanin (8) | 7.96 ± 0.76 e | 4.56 ± 0.63 e |
| Sakuranetin (9) | 1.74 ± 0.17 e | (23.84 ± 4.91) d |
| 6,7-Dimethoxy-2-(2-phenylethyl)chromone (10) | 11.54 ± 2.19 e | 10.48 ± 1.35 d |
| (–)-Syringaresinol (11) | (30.23 ± 1.71) e | (25.12 ± 6.22) d |
| Taraxacine A (12) | (14.64 ± 2.95) d | (44.43 ± 1.90) e |
| Methyl 3,4-dihydroxybenzoate (13) | (1.32 ± 2.25) | (19.13 ± 5.85) c |
| Vanillic acid (14) | 29.34 ± 6.01 e | 29.92 ± 2.50 e |
| Docosyl caffeate (15) | (1.23 ± 5.08) | (25.57 ± 5.00) d |
| Docosyl trans-ferulate (16) | (27.83 ± 4.37) | (27.12 ± 6.23) |
| β-Sitostenone (17) | (2.74 ± 0.96) c | (3.92 ± 2.22) |
| β-Sitosterol (18) | (9.08 ± 6.13) | (2.43 ± 2.95) |
| Ergosta-4,6,8(14),22-tetraen-3-one (19) | (42.63 ± 1.82) d | 15.25 ± 3.75 c |
| trans-Phytol (20) | (4.91 ± 5.52) | (22.65 ± 5.66) d |
| α-Tocopherol (21) | (0.55 ± 2.51) | (9.37 ± 4.92) |
| α-Tocospiro A (22) | (0.55 ± 2.51) | (9.37 ± 4.92) |
| Blumenol A (23) | (1.37 ± 1.38) | (10.72 ± 1.62) d |
| 2,6-Dimethoxy-p-benzoquinone (24) | (47.09 ± 2.85) e | (16.00 ± 5.53) c |
| Diphenyleneiodonium | 1.73 ± 0.72 e | – |
| Phenylmethylsulfonyl fluoride | – | 199.6 ± 30.7 e |
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. General Experimental Procedures
4.3. Plant Material
4.4. Extraction and Isolation
4.5. Biological Assay
4.5.1. Preparation of Human Neutrophils
4.5.2. Measurement of Superoxide Anion Generation
4.5.3. Measurement of Elastase Release
4.5.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, T.F.; Huang, Z.S. Colour Atlas of Herbal Drugs in China; Guang-dong Science & Technology Press: Guangzhou, China, 2011; p. 421. [Google Scholar]
- Pranakhon, R.; Pannangpetch, P.; Aromdee, C. Antihyperglycemic activity of agarwood leaf extracts in STZ-induced diabetic rats and glucose uptake enhancement activity in rat adipocytes. Songklanakarin J. Sci. Technol. 2011, 33, 405–410. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl)chromone derivatives. Acta Pharm. Sin. 1989, 24, 678–683. [Google Scholar]
- Lin, L.D.; Qi, S.Y. Triterpenoid from Chinese eaglewood (Aquilaria sinensis). Chin. Tradit. Herb. Drugs 2000, 31, 89–90. [Google Scholar]
- Wang, H.G.; Zhou, M.H.; Lu, J.J.; Yu, B.Y. Antitumor constituents from the leaves of Aquilaria sinensis (Lour.) Gilg. Chem. Ind. For. Prod. 2008, 28, 1–5. [Google Scholar]
- Nie, C.X.; Song, Y.L.; Chen, D.; Xue, P.F.; Tu, P.F.; Wang, K.Y.; Chen, J.M. Studies on chemical constituents of leaves of Aquilaria sinensis. Zhongguo Zhongyao Zazhi 2009, 34, 858–860. [Google Scholar] [PubMed]
- Wang, Q.H.; Peng, K.; Tan, L.H.; Dai, H.F. Aquilarin A, a new benzenoid derivative from the fresh stem of Aquilaria sinensis. Molecules 2010, 15, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Qi, J.; Zhu, D.N.; Yu, B.Y. Antioxidant activity and structure-activity relationship of the flavones from the leaves of Aquilaria sinensis. Chin. J. Nat. Med. 2008, 6, 456–460. [Google Scholar] [CrossRef]
- Qi, J.; Lu, J.J.; Liu, J.H.; Yu, B.Y. Flavonoid and a rare benzophenone glycoside from the leaves of Aquilaria sinensis. Chem. Pharm. Bull. 2009, 57, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.F.; Liu, J.; Han, Z.; Zeng, Y.B.; Wang, H.; Mei, W.L. Two new 2-(2-phenylethyl)chromones from Chinese eaglewood. J. Asian Nat. Prod. Res. 2010, 12, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Mei, W.L.; Wu, J.; Dai, H.F. Flavones from stem of Aquilaria sinensis. J. Trop. Subtrop. Bot. 2010, 18, 97–100. [Google Scholar]
- Yang, X.B.; Feng, J.; Yang, X.W.; Zhao, B.; Liu, J.X. Aquisiflavoside, a new nitric oxide production inhibitor from the leaves of Aquilaria sinensis. J. Asian Nat. Prod. Res. 2012, 14, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.X.; Liang, Y.G.; Lu, Q.L. Isolation of apigenin-7,4′-dimethylethers from leaves of Aquilaria sinensis (Lour.) Gilg by high-speed counter-current chromatography and its scavenging effect on nitrite in vitro. J. Anhui Agric. Sci. 2013, 41, 6648–6650. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L.; He, C.H.; Zheng, Q.T.; Yang, J. Chemical constituents of Aquilaria sinensis (Lour.) Gilg. III. Elucidation of the structure of isobaimuxinol and isolation and identification of the constituents of lower boiling point fraction of the volatile oil. Acta Pharm. Sin. 1989, 24, 264–268. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria. sinensis (Lour.) Gilg. V. Isolation and characterization of three 2-(2-phenylethyl)chromone derivatives. Acta Pharm. Sin. 1990, 25, 186–190. [Google Scholar]
- Yagura, T.; Ito, M.; Kiuchi, F.; Honda, G.; Shimada, Y. Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem. Pharm. Bull. 2003, 51, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, B.; Zeng, Y.E. Determination of 2 active components in Aquilaria sinensis (Lour.) Gilg by HPLC. Shizhen Guoyi Guoyao 2007, 18, 1697–1698. [Google Scholar]
- Liu, J.; Wu, J.; Zhao, Y.X.; Deng, Y.Y.; Mei, W.L.; Dai, H.F. A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin. Chem. Lett. 2008, 19, 934–936. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Z.R.; Chai, X.Y.; Zeng, K.W.; Jia, Y.X.; Bi, D.; Ma, Z.Z.; Tu, P.F. Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur. J. Org. Chem. 2012, 2012, 5389–5397. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.W. Liposolubility constituents from leaves of Aquilaria sinensis. China J. Chin. Mater. Med. 2011, 36, 2092–2095. [Google Scholar]
- Yang, L.; Qiao, L.R.; Xie, D.; Dai, J.G.; Guo, S.X. Sesquiterpenes and monoterpene from Aquilaria sinensis. China J. Chin. Mater. Med. 2012, 37, 1973–1976. [Google Scholar]
- Lin, F.; Mei, W.L.; Zuo, W.J.; Peng, K.; Dai, H.F. Chemical constituents from fruits of Aquilaria sinensis. J. Trop. Subtrop. Bot. 2012, 20, 89–91. [Google Scholar]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Abdellatif, Z.; Akino, J.; Bernard, B. DNA topoisomerase I inhibitors: Cytotoxic flavones from Lethedon tannaensis. J. Nat. Prod. 1996, 59, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Sun, J.; Jiang, Y.; Tu, P.F. Further chemical investigation of leaves of Aquilaria sinensis. China J. Chin. Mater. Med. 2013, 38, 3299–3303. [Google Scholar]
- Nguyen, T.K.P.; Nguyen, K.P.P.; Kamounah, F.S.; Zhang, W.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Takae, T.; Tenji, K.; Shiu, K. Studies on the agarwood I. Structures of 2-(2-phenylethyl)chromone derivatives. Chem. Pharm. Bull. 1982, 30, 3791–3795. [Google Scholar] [CrossRef]
- Tsakou, O.; Skaltsa, H.; Harvala, C. Flavonoids from Achillea crithmifolia Waldst & Kit. Sci. Pharm. 1996, 64, 197–202. [Google Scholar]
- Righi, G.; Silvestri, I.P.; Barontini, M.; Crisante, F.; di Manno, A.; Pelagalli, R.; Bovicelli, P. Efficient synthesis of scutellarein. Nat. Prod. Res. 2012, 26, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.M.; Xiao, J.; Li, X.; Wang, J.H. Isolation and identification of flavonoids from buds of Daphne genkwa Sieb. et Zucc. Processed by rice vinegar. J. Shenyang Pharm. Univ. 2009, 26, 353–356. [Google Scholar]
- Jerz, G.; Waibel, R.; Achenbach, H. Cyclohexanoid protoflavanones from the stem-bark and roots of Ongokea gore. Phytochemistry 2005, 66, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.W.; Harrison, L.J.; Powell, A.D. Phenanthrene and other aromatic constituents of Bulbophyllum vaginatum. Phytochemistry 1999, 50, 1237–1241. [Google Scholar] [CrossRef]
- Leu, Y.L.; Shi, L.S.; Damu, A.G. Chemical constituents of Taraxacum formosanum. Chem. Pharm. Bull. 2003, 51, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Makhmoor, T.; Choudhary, M.I. Radical scavenging potential of compounds isolated from Vitex agnus-castus. Turk. J. Chem. 2010, 34, 119–126. [Google Scholar]
- Yang, Y.; Jiang, J.; Qimei, L.; Yan, X.; Zhao, J.; Yuan, H.; Qin, Z.; Wang, M. The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules 2010, 15, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhong, Y.; Zuo, C.X.; Yin, J.T.; Wang, B. Isolation and structure identification of the chemical constituents from Gypsophila oldhamiana. Acta Pharm. Sin. 2005, 40, 994–996. [Google Scholar]
- Zhao, Y.; Jia, Z.; Yang, L. Sinapyl alcohol derivatives and other constituents from Ligularia nelumbifolia. Phytochmtstry 1994, 37, 1149–1152. [Google Scholar] [CrossRef]
- Cui, J.G.; Fan, L.; Huang, L.L.; Liu, H.L.; Zhou, A.M. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (I). Steroids 2009, 74, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Shue, M.J. New esters, 2-(4-hydroxy-3-methoxyphenyl)ethyl hexa- and octacosanoates from the leaves of Cinnamomum reticulatum Hay. J. Chin. Chem. Soc. 1991, 38, 65–69. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, Y.; Ahn, J.K.; Park, S.Y.; Lee, H.J. Cytotoxic activity of ergosta-4,6,8(14),22-tetraen-3-one from the sclerotia of Polyporus umbellatus. Bull. Korean Chem. Soc. 2005, 26, 1464–1466. [Google Scholar] [CrossRef]
- Ki, H.K.; Kyu, H.L.; Sang, U.C.; Young, H.K.; Kang, R.L. Terpene and phenolic constituents of Lactuca indica L. Arch. Pharm. Res. 2008, 31, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.L.; Jeng, Y.F.; Duh, C.Y.; Chen, I.S. Cytotoxic constituents from the leaves of Litsea akoensis. J. Chin. Pharm. Sci. 2001, 53, 291–301. [Google Scholar]
- Chiang, Y.M.; Kuo, Y.H. Two novel α-tocopheroids from the aerial roots of Ficus microcarpa. Tetrahedron Lett. 2003, 44, 5125–5128. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The constituents of Lindera glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stem of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981, 17, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Leu, Y.L.; Kao, S.H.; Tang, M.C.; Chang, H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ting, C.W.; Wu, Y.C.; Hwang, T.L.; Cheng, M.J.; Sung, P.J.; Wang, T.C.; Chen, J.F. New labdane-type diterpenoids and anti-inflammatory constituents from Hedychium coronarium. Int. J. Mol. Sci. 2013, 14, 13063–13077. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Hwang, T.-L.; Chung, M.-I.; Sung, P.-J.; Shu, C.-W.; Cheng, M.-J.; Chen, J.-J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis. Molecules 2015, 20, 20912-20925. https://doi.org/10.3390/molecules201119736
Wang S-L, Hwang T-L, Chung M-I, Sung P-J, Shu C-W, Cheng M-J, Chen J-J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis. Molecules. 2015; 20(11):20912-20925. https://doi.org/10.3390/molecules201119736
Chicago/Turabian StyleWang, Sin-Ling, Tsong-Long Hwang, Mei-Ing Chung, Ping-Jyun Sung, Chih-Wen Shu, Ming-Jen Cheng, and Jih-Jung Chen. 2015. "New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis" Molecules 20, no. 11: 20912-20925. https://doi.org/10.3390/molecules201119736