An Efficient and Recyclable Nanoparticle-Supported Cobalt Catalyst for Quinoxaline Synthesis
Abstract
:1. Introduction

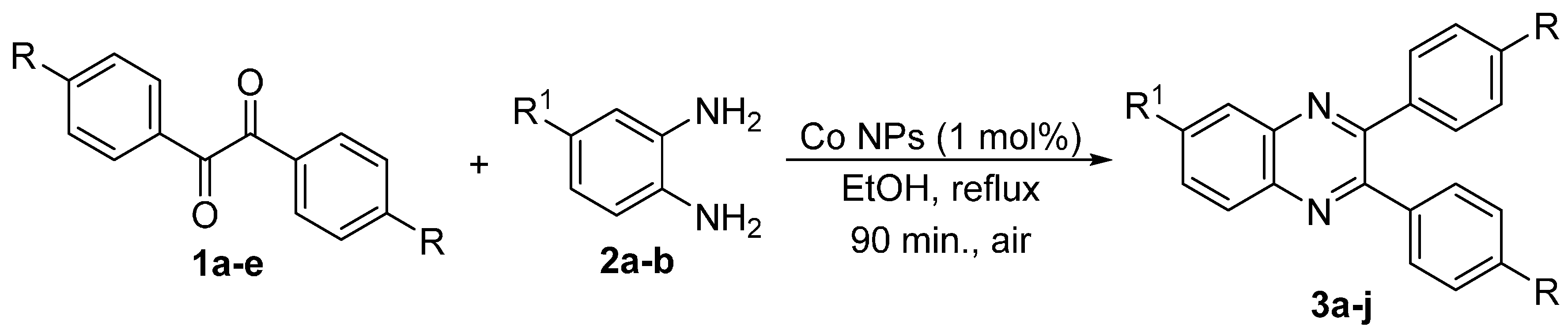
2. Results and Discussion
| Entry | Catalyst (mol %) | Solvent | Temperature (°C) | Time (h) | Yield 3a (%) b |
|---|---|---|---|---|---|
| 1 | - | - | 100 | 2 | - |
| 2 | 2 | H2O | 100 | 2 | 87 |
| 3 | 2 | H2O | 50 | 2 | 57 |
| 4 | 1 | H2O | 100 | 2 | 86 |
| 5 | 2 | EtOH | 78 | 2 | 93 |
| 6 | 2 | EtOH | 50 | 2 | 60 |
| 7 | 1 | EtOH | 78 | 2 | 93 |
| 8 | 1 | EtOH | 78 | 1.5 | 92 |
| 9 | 0.5 | EtOH | 78 | 1.5 | 80 |
| 10 | 1 | - | 100 | 2 | 72 |
| Entry | 1,2-Diketone 1 | 1,2-Diamines 2 | Product 3 | Yield (%) b | M.P. (°C) |
|---|---|---|---|---|---|
| 1 |  1a |  2a |  3a | 92 | 188–190 |
| 2 |  1b | 2a |  3b | 92 | 195–197 |
| 3 |  1c | 2a |  3c | 92 | 173–175 |
| 4 |  1d | 2a |  3d | 90 | 175–177 |
| 5 |  1e | 2a |  3e | 88 | 143–145 |
| 6 |  1a |  2b |  3f | 92 | 127–129 |
| 7 |  1b | 2b |  3g | 96 | 127–129 |
| 8 |  1c | 2b |  3h | 94 | 133–135 |
| 9 |  1d | 2b |  3i | 94 | 190–192 |
| 10 |  1e | 2b |  3j | 94 | 134–135 |
| 11 |  |  2c |  3k | 98 | 116–117 |
| 12 |  | 2c |  3l | 95 | 163–165 |
| Run No. a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Yield (%) b | 94 | 94 | 92 | 92 | 92 | 90 | 91 | 90 | 90 | 87 |
| Entry | Condition | Time (min) | Yield (%) | Reference |
|---|---|---|---|---|
| 1 | Polyaniline-sulfate salt (5 wt %), DCE, r.t. | 20 | 95 | [25] |
| 2 | CAN (5 mol %), H2O, r.t. | 10 | 98 | [26] |
| 3 | I2 (10 mol %), DMSO, r.t. | 35 | 95 | [27] |
| 4 | MeOH:AcOH (9:1), MW, 160 °C | 5 | 99 | [28] |
| 5 | Acidic alumina, 80 °C | 2 | 96 | [29] |
| 6 | Citric acid (10 mol %), EtOH, r.t. | 1 | 94 | [30] |
| 7 | Fe3O4NPs (10 mol %), H2O, r.t. | 150 | 95 | [31] |
| 8 | Silicabonded S-sulfonicacid (3.4 mol %), EtOH/H2O (70/30), r.t. | 5 | 96 | [32] |
| 9 | Ga(OTf)3 (1 mol %), EtOH, r.t. | 5 | 99 | [33] |
| 10 | Bi(OTf)3 (10 mol %), H2O, r.t. | 5 | 97 | [34] |
| 11 | CoNP (1mol %), EtOH, reflux | 90 | 92 | Our work |
3. Experimental Section
3.1. General Information
3.2. Preparation of the Supported Cobalt Catalyst
3.3. General Reaction Procedure
3.4. Selected Spectroscopic Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, D.J. Quinoxalines: Supplement II. In The Chemistry of Heterocyclic Compounds; John Wiley & Sons: Princeton, NJ, USA, 2004. [Google Scholar]
- Katritzky, A.R.; Pozharskii, A.F. Handbook of Heterocyclic Chemistry; Pergamon: Oxford, UK, 2000. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Moorthy, N.; Hari, N.S.; Manivannan, E.; Karthikeyan, C.; Trivedi, P. 6H-Indolo[2,3-b]quinoxalines: DNA and protein interacting scaffold for pharmacological activities. Mini Rev. Med. Chem. 2013, 13, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Rong, F.; Chow, S.; Yan, S.; Larson, G.; Hong, Z.; Wu, J. Structure–activity relationship (SAR) studies of quinoxalines as novel HCV NS5B RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.A.; Lim, H.D.; Hanzer, A.; Zuiderveld, O.P.; Guaita, E.; Adami, M.; Coruzzi, G.; Leurs, R.; Esch, I.J.P. Fragment based design of new H4 receptor-ligands with anti-inflammatory properties in vivo. J. Med. Chem. 2008, 51, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, S.T.; Polin, L.; Kushner, J.; Paluch, J.; White, K.; Edelstein, M.; Palomino, E.; Corbett, T.H.; Horwitz, J.P. Design, synthesis, and biological evaluation of analogues of the antitumor agent, 2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid (XK469). J. Med. Chem. 2001, 44, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Rajule, R.; Bryant, V.C.; Lopez, H.; Luo, X.; Natarajan, A. Perturbing pro-survival proteins using quinoxaline derivatives: A structure–activity relationship study. Bioorg. Med. Chem. 2012, 20, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Desrivot, J.; Bories, C.; Loiseau, P.M.; Franck, X.; Hocquemiller, R.; Figadere, B. Synthesis and antiprotozoal activity of some new synthetic substituted quinoxalines. Bioorg. Med. Chem. Lett. 2006, 16, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, Y.H.; Park, J.Y.; Kim, S.K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Maeda, H.; Mizuno, T.; Lynch, V.M.; Furuta, H. Quinoxaline-Bridged Porphyrinoids. J. Am. Chem. Soc. 2002, 124, 13474–13479. [Google Scholar] [CrossRef] [PubMed]
- Azov, V.A.; Beeby, A.; Cacciarini, M.; Cheetham, A.G.; Diederich, F.; Frei, M.; Gimzewski, J.K.; Gramlich, V.; Hecht, B.; Jaun, B.; et al. Resorcin[4]arenecavitand-based molecular switches. Adv. Funct. Mater. 2006, 16, 147–156. [Google Scholar] [CrossRef]
- Champion, R.D.; Cheng, K.-F.; Pai, C.-L.; Chen, W.-C.; Jenekhe, S.A. Electronic properties and field-effect transistors of thiophene-based donor–acceptor conjugated copolymers. Macromol. Rapid Commun. 2005, 26, 1835–1840. [Google Scholar] [CrossRef]
- Wang, E.; Hou, L.; Wang, Z.; Hellström, S.; Zhang, F.; Inganäs, O.; Andersson, M.R. An easily synthesized blue polymer for high-performance polymer solar cells. Adv. Mater. 2010, 22, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Lee, H.J.; Kim, J.H.; Park, S.Y.; Park, S.-M.; Dai, L.; Baek, J.-B. Novel quinoxaline-based organic sensitizers for dye-sensitized solar cells. Org. Lett. 2011, 13, 3880–3883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shim, J.W.; Tiwari, S.P.; Zhang, Q.; Norton, J.E.; Wu, P.-T.; Barlow, S.; Jenekhe, S.A.; Kippelen, B.; Brédas, J.-L.; et al. Dithienopyrrole–quinoxaline/pyridopyrazinedonor-acceptorpolymers: Synthesis and electrochemical, optical, charge-transport, and photovoltaic properties. J. Mater. Chem. 2011, 21, 4971–4982. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhen, H.; Kroon, R.; Tang, Z.; Hellström, S.; Hou, L.; Wang, E.; Gedefaw, D.; Inganäs, O.; Zhang, F.; et al. Molecular orbital energy level modulation through incorporation of selenium and fluorine into conjugated polymers for organic photovoltaic cells. J. Mater. Chem. A 2013, 1, 13422–13425. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.-G.; Fu, Q.; Li, Y. Synthesis and photovoltaic properties of a D–A copolymer based on the 2,3-di(5-hexylthiophen-2-yl)quinoxaline acceptor unit. Macromol. Chem. Phys. 2014, 215, 597–603. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Zhang, J.; Gu, J.; Zhang, L. Synthesis of two conjugated polymers as TNT chemosensor materials. Sens. Actuators B 2010, 149, 155–160. [Google Scholar] [CrossRef]
- Brock, E.D.; Lewis, D.M.; Yousaf, T.I.; Harper, H.H. Reactive Dye Compounds. U.S. Patent WO1999051688 A1, 14 October 1999. [Google Scholar]
- Lindner, B.D.; Zhang, Y.; Hoefle, S.; Berger, N.; Teusch, C.; Jesper, M.; Hardcastle, K.I.; Qian, X.; Lemmer, U.; Colsmann, A.; et al. N-fused quinoxalines and benzoquinoxalines as attractive emitters for organic light emitting diodes. J. Mater. Chem. C 2013, 1, 5718–5724. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Velusamy, M.; Lin, J.T.; Chuen, C.-H.; Tao, Y.-T. Chromophore-labeled quinoxaline derivatives as efficient electroluminescent materials. Chem. Mater. 2005, 17, 1860–1866. [Google Scholar] [CrossRef]
- Ayaz, M.; Xu, Z.; Hulme, C. Novel succinct routes to quinoxalines and 2-benzimidazolylquinoxalines via the Ugi reaction. Tetrahedron Lett. 2014, 55, 3406–3409. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, C.; Kumar, C.N.S.S.P.; Rao, V.J.; Palaniappan, S. Efficient, convenient and reusable polyaniline-sulfate salt catalyst for the synthesis of quinoxaline derivatives. J. Mol. Catal. A Chem. 2007, 265, 227–230. [Google Scholar] [CrossRef]
- More, S.V.; Sastry, M.N.V.; Yao, C.-F. Cerium(IV) ammonium nitrate(CAN) as a catalyst in tap water: A simple, proficient and green approach for the synthesis of quinoxalines. Green Chem. 2006, 8, 91–95. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Sarda, S.R.; Ardhapure, S.S.; Jadhav, W.N.; Bhusare, S.R.; Pawar, R.P. An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using molecular iodine as the catalyst. Tetrahedron Lett. 2005, 46, 7183–7186. [Google Scholar] [CrossRef]
- Zhao, Z.; Wisnoski, D.D.; Wolkenberg, S.E.; Leister, W.H.; Wang, Y.; Lindsley, C.W. General microwave-assisted protocols for the expedient synthesis of quinoxalines and heterocyclic pyrazines. Tetrahedron Lett. 2004, 45, 4873–4876. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Danehchin, M. Easy access to quinoxaline derivatives using alumina as an effective and reusable catalyst under solvent-free conditions. Appl. Catal. A Gen. 2011, 394, 48–51. [Google Scholar] [CrossRef]
- Mahesh, R.; Dhar, A.K.; Sasank, T.V.N.V.T.; Thirunavukkarsu, S.; Devadoss, T. Citric acid: An efficient and green catalyst for rapid one pot synthesis of quinoxaline derivatives at room temperature. Chin. Chem. Lett. 2011, 22, 389–392. [Google Scholar] [CrossRef]
- Lü, H.-Y.; Yang, S.-H.; Deng, J.; Zhang, Z.-H. Magnetic Fe3O4 nanoparticles as new, efficient, and reusable catalysts for the synthesis of quinoxalines in water. Aust. J. Chem. 2010, 63, 1290–1296. [Google Scholar] [CrossRef]
- Niknam, K.; Saberi, D.; Mohagheghnejad, M. Silica bonded S-sulfonic acid: A recyclable catalyst for the synthesis of quinoxalines at room temperature. Molecules 2009, 14, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-J.; Zou, J.-P.; Pan, X.-Q.; Zhang, W. Gallium(III) triflate-catalyzed synthesis of quinoxaline derivatives. Tetrahedron Lett. 2008, 49, 7386–7390. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Premlatha, K.; Shankar, K.S. Bismuth(III)-catalyzed rapid synthesis of 2,3-disubstituted quinoxalines in water. Synthesis 2008, 3787–3792. [Google Scholar] [CrossRef]
- Hille, T.; Irrgang, T.; Kempe, R. The Synthesis of benzimidazoles and quinoxalines from aromatic diamines and alcohols by iridium-catalyzed acceptorless dehydrogenative alkylation. Chem. Eur. J. 2014, 20, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Oh, S.G. A new ruthenium-catalyzed approach for quinoxalines from o-phenylenediamines and vicinal-diols. Tetrahedron Lett. 2006, 47, 5633–5636. [Google Scholar] [CrossRef]
- Jeena, V.; Robinson, R.S. An environmentally friendly, cost effective synthesis of quinoxalines: The influence of microwave reaction conditions. Tetrahedron Lett. 2014, 55, 642–645. [Google Scholar] [CrossRef]
- Sithambaram, S.; Ding, Y.; Li, W.; Shen, X.; Gaenzler, F.; Suib, S.L. Manganese octahedral molecular sieves catalyzed tandem process for synthesis of quinoxalines. Green Chem. 2008, 10, 1029–1032. [Google Scholar] [CrossRef]
- Robinson, R.S.; Taylor, R.J.K. Quinoxaline synthesis from α-hydroxy ketones via a tandem oxidation process using catalysed aerobic oxidation. Synlett 2005, 1003–1005. [Google Scholar]
- Kim, S.Y.; Park, K.H.; Chung, Y.K. Manganese(IV) dioxide-catalyzed synthesis of quinoxalines under microwave irradiation. Chem. Commun. 2005, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Raw, S.A.; Wilfred, C.D.; Taylor, R.J.K. Tandem oxidation processes for the preparation of nitrogen-containing heteroaromatic and heterocyclic compounds. Org. Biomol. Chem. 2004, 2, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, D.; Attanasi, O.A.; Filippone, P.; Ignacio, R.; Lillini, S.; Mantellini, F.; Palacios, F.; Santos, J.M. Straightforward access to pyrazines, piperazinones, and quinoxalinesby reactions of 1,2-diaza-1,3-butadienes with 1,2-diamines under solution, solvent-free, or solid-phase conditions. J. Org. Chem. 2006, 71, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Grau, D.; Hampel, F.; Tsogoeva, S.B. α-Nitro epoxides in organic synthesis: development of a one-pot organocatalytic strategy for the synthesis of quinoxalines. Eur. J. Org. Chem. 2014, 1401–1405. [Google Scholar] [CrossRef]
- Antoniotti, S.; Dunach, E. Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1,2-diamines. Tetrahedron Lett. 2002, 43, 3971–3973. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P.; Al-Mourabit, A. A simple and straightforward approach to quinoxalines by iron/sulfur-catalyzed redox condensation of o-nitroanilines and phenethylamines. Org. Lett. 2013, 15, 5238–5241. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, X. Ruthenium-catalyzed oxidation of alkynes to 1,2-diketones under room temperature and one-pot synthesis of quinoxalines. Tetrahedron Lett. 2013, 54, 642–645. [Google Scholar] [CrossRef]
- Shi, S.; Wang, T.; Yang, W.; Rudolph, M.; Hashmi, A.S.K. Gold-catalyzed synthesis of glyoxals by oxidation of terminal alkynes: one-pot synthesis of quinoxalines. Chem. Eur. J. 2013, 19, 6576–6580. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Takeda, Y.; Kiyokawa, K.; Minakata, S. Hypervalent iodine(III)-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes: Direct synthesis of quinoxalines from alkyne substrates under metal-free conditions. Chem. Commun. 2013, 49, 9266–9268. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Hu, W.-P.; Liu, M.-C.; Yan, P.-C.; Wang, J.-J.; Chung, M.-I. Efficient synthesis of quinoxalines with hypervalent iodine as a catalyst. Tetrahedron 2013, 69, 9735–9741. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Zhang, L.; Jiao, N. Et3N-catalyzed oxidative dehydrogenative coupling of α-unsubstituted aldehydes and ketones with aryl diamines leading to quinoxalines using molecular oxygen as oxidant. Tetrahedron 2012, 68, 5258–5262. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Luque, R.; Budarin, V.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Feiz, A.; Luque, R. An efficient synthesis of coumarin derivatives using a SBA-15 supported Cobalt(II) nanocatalyst. Catal. Lett. 2015, 145, 1621–1625. [Google Scholar] [CrossRef]
- Rajabi, F.; Raessi, M.; Arancon, R.A.D.; Saidi, M.R.; Luque, R. Supported cobalt oxide nanoparticles as efficient catalyst in esterification and amidation reactions. Catal. Commun. 2015, 59, 122–126. [Google Scholar] [CrossRef]
- Heravi, M. M.; Taheri, S.; Bakhtiari, K.; Oskooie, H. A. Zn[(L)proline]: A powerful catalyst for the very fast synthesis of quinoxaline derivatives at room temperature. Catal. Commun. 2007, 8, 1341–1344. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3a–l are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, F.; Alves, D.; Luque, R. An Efficient and Recyclable Nanoparticle-Supported Cobalt Catalyst for Quinoxaline Synthesis. Molecules 2015, 20, 20709-20718. https://doi.org/10.3390/molecules201119731
Rajabi F, Alves D, Luque R. An Efficient and Recyclable Nanoparticle-Supported Cobalt Catalyst for Quinoxaline Synthesis. Molecules. 2015; 20(11):20709-20718. https://doi.org/10.3390/molecules201119731
Chicago/Turabian StyleRajabi, Fatemeh, Diego Alves, and Rafael Luque. 2015. "An Efficient and Recyclable Nanoparticle-Supported Cobalt Catalyst for Quinoxaline Synthesis" Molecules 20, no. 11: 20709-20718. https://doi.org/10.3390/molecules201119731









