Temporal Variation of Aristolochia chilensis Aristolochic Acids during Spring
Abstract
:1. Introduction
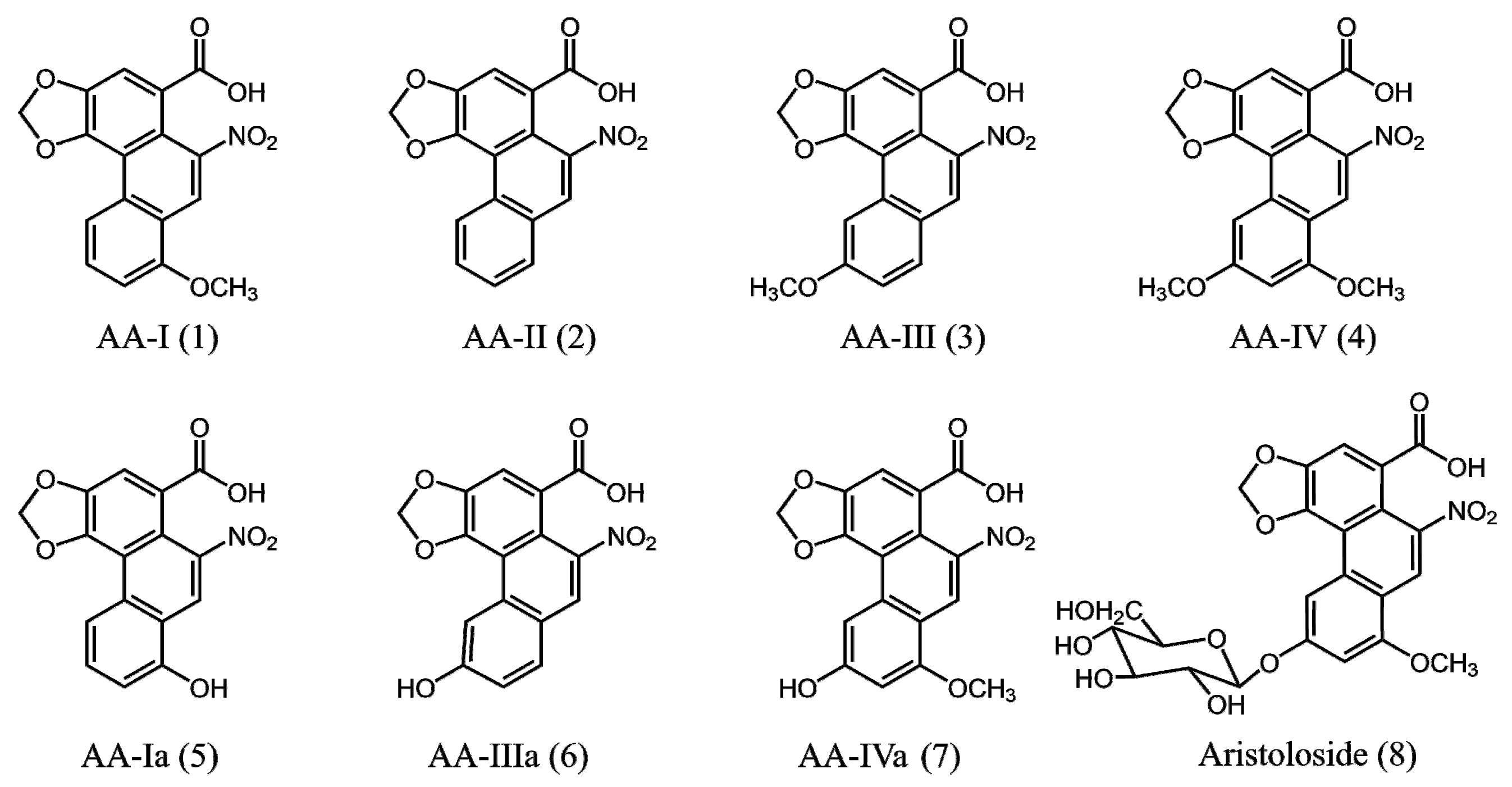
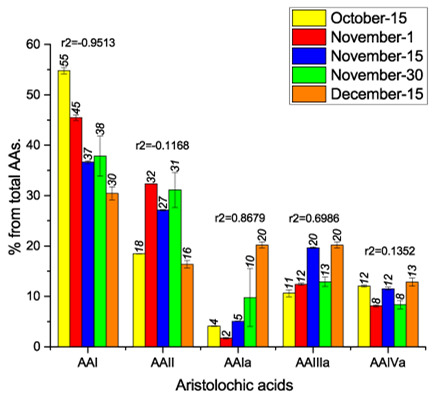
2. Results and Discussion
| Non Phenolic Acids | Phenolic Acids | |||||
|---|---|---|---|---|---|---|
| Harvest Date | AA-I | AA-II | AA-Ia | AA-IIIa | AA-IVa | Total AAs |
| October-15 | 212.6 ± 3.8 | 71.6 ± 2.2 | 15.9 ± 0.8 | 41.2 ± 3.7 | 46.7 ± 0.8 | 388.0 ± 5.9 |
| November-1 | 206.3 ± 5.8 | 146.8 ± 5.9 | 7.7 ± 1.0 | 56.3 ± 3.2 | 36.9 ± 2.4 | 453.9 ± 9.2 |
| November-15 | 195.5 ± 2.8 | 144.6 ± 2.4 | 26.9 ± 0.3 | 104.9 ± 3.2 | 61.5 ± 2.8 | 533.5 ± 5.7 |
| November-30 | 154.5 ± 0.8 | 129.7 ± 3.1 | 61.9 ± 8.1 | 49.4 ± 9.5 | 31.4 ± 4.7 | 426.9 ± 13.7 |
| December-15 | 145.6 ± 1.2 | 78.3 ± 0.5 | 96.8 ± 7.8 | 96.8 ± 7.8 | 61.6 ± 7.1 | 479.1 ± 13.2 |
| Non Phenolic Acids | Phenolic Acids | |||||
|---|---|---|---|---|---|---|
| Harvest Date | AA-I | AA-II | AA-Ia | AA-IIIa | AA-IVa | Total-AAs |
| October-15 | 104.6 ± 3.2 | 20.6 ± 0.7 | - | 12.3 ± 2.5 | 5.4 ± 1.0 | 142.8 ± 4.2 |
| November-1 | 307.5 ± 3.0 | 68.4 ± 1.5 | - | 39.2 ± 2.0 | 23.7 ± 2.2 | 438.8 ± 4.5 |
| November-15 | 325.3 ± 8.2 | 118.6 ±3.7 | - | 49.3 ± 2.0 | 47.1 ± 2.9 | 540.3 ± 9.7 |
| November-30 | 294.6±10.6 | 102.1 ±2.9 | 8.8 ± 0.5 | 41.2 ± 3.0 | 39.4 ± 3.0 | 486.1 ± 11.8 |
| December-15 | 265.4 ± 3.7 | 65.5 ± 3.4 | 46.9 ± 3.6 | 90.6 ± 9.2 | 90.6 ± 9.8 | 559.0 ± 14.8 |

3. Experimental Section
3.1. Plant Material
3.2. Extract Preparation
3.3. HPLC Analysis of AAs

3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michl, J.; Ingrouille, M.J.; Simmonds, M.S.; Heinrich, M. Naturally occurring aristolochic acid analogues and their toxicities. Nat. Prod. Rep. 2014, 31, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Chan, J.; Wanke, S.; Neinhuis, C.; Simmonds, M.S. Local uses of aristolochia species and content of nephrotoxic aristolochic acid 1 and 2—A global assessment based on bibliographic sources. J. Ethnopharm. 2009, 125, 108–144. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, P.; Aldunate, G.F. Flora Nativa de Valor Ornamental: Chile Zona Centro, 1st ed.; Editorial Andrés Bello: Santiago, Chile, 2001; Volume 1, pp. 344–345. [Google Scholar]
- Zin, J.; Weiss, C. La Salud Por Medio de Plantas Medicinales, 8th ed.; Editorial Don Bosco: Santiago, Chile, 1998; p. 148. [Google Scholar]
- Salazar, C. La maldición de las orejas de zorro, llega a su fin. Urbatorivm 2013. Available online: http://urbatorium.blogspot.cl/2013/09/la-maldicion-de-las-orejas-de-zorro.html (accessed on 6 September 2015).
- Wilhelm de Möesbach, E. Botánica Indígena de Chile, 1st ed.; Editorial Andrés Bello: Santiago, Chile, 1992; p. 103. [Google Scholar]
- Muñoz, M.; Barrera, E.; Meza, I. El Uso medicinal y alimenticio de las plantas nativas y naturalizadas en Chile. Pub. Ocas. Mus. Nac. Hist. Nat. 1981, 33, 3–91. [Google Scholar]
- Urzúa, A.; Olguín, A.; Santander, R. Aristolochic acids in the roots of aristolochia chilensis, a dangerous Chilean medicinal plant. J. Chil. Chem. Soc. 2013, 58, 2089–2091. [Google Scholar] [CrossRef]
- Urzúa, A.; Santander, R.; Sotes, G. Aristolochic acids from aristolochia bridgesii, a host-plant of Battus polydamas archidamas. J. Chil. Chem. Soc. 2009, 54, 437–438. [Google Scholar] [CrossRef]
- Balachandran, P.; Wei, F.; Lin, R.C.; Khan, I.A.; Pasco, D.S. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Kidney Int. 2005, 67, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Shang, M.; Yu, J.; Xu, Y.; Li, Z.; Lei, L.; Li, X.; Cai, S.; Namba, T. Simultaneous determination of five aristolochic acids and two aristololactams in aristolochia plants by high-performance liquid chromatography. Biomed. Chromatogr. 2006, 20, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Nie, L.; Zeng, D.; Luo, X.; Tang, F.; Ding, L.; Liu, Q.; Guo, M.; Yao, S. Simultaneous determination of nine aristolochic acid analogues in medicinal plants and preparations by high-performance liquid chromatography. Talanta 2007, 73, 644–665. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Q.; Zhu, W.; Ding, L.; Tang, F.; Yao, S. Simultaneous analysis of six aristolochic acids and five aristolactams in herbal plants and their preparations by high-performance liquid chromatography-diode array detection-fluorescence detection. J. Chromatogr. A 2008, 1182, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Li, X.; Chen, J.W.; Guo, S.; Cai, B.C. Simultaneous determination of eleven bioactive compounds in Saururus chinensis from different harvesting seasons by HPLC-DAD. J. Pharm. Biomed. Anal. 2010, 51, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.; Whiley, L.; Hong, Y.; Sebastian, C.M.; Smith, N.W.; Legido-Quigley, C. Evaluation of Chinese medicinal herbs fingerprinting by HPLC-DAD for the detection of toxic aristolochic acids. J. Sep. Sci. 2011, 34, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.F.; Urzúa, A.; Niemeyer, H.M. Sequestration of aristolochic acids from meridic diets by larvae of Battus polydamas archidamas (Papilionidae: troidini). Eur. J. Entomol. 2011, 108, 41–45. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santander, R.; Urzúa, A.; Olguín, Á.; Sánchez, M. Temporal Variation of Aristolochia chilensis Aristolochic Acids during Spring. Molecules 2015, 20, 20391-20396. https://doi.org/10.3390/molecules201119704
Santander R, Urzúa A, Olguín Á, Sánchez M. Temporal Variation of Aristolochia chilensis Aristolochic Acids during Spring. Molecules. 2015; 20(11):20391-20396. https://doi.org/10.3390/molecules201119704
Chicago/Turabian StyleSantander, Rocío, Alejandro Urzúa, Ángel Olguín, and María Sánchez. 2015. "Temporal Variation of Aristolochia chilensis Aristolochic Acids during Spring" Molecules 20, no. 11: 20391-20396. https://doi.org/10.3390/molecules201119704