Pharmacokinetics of Ginkgolide B after Oral Administration of Three Different Ginkgolide B Formulations in Beagle Dogs
Abstract
:1. Introduction
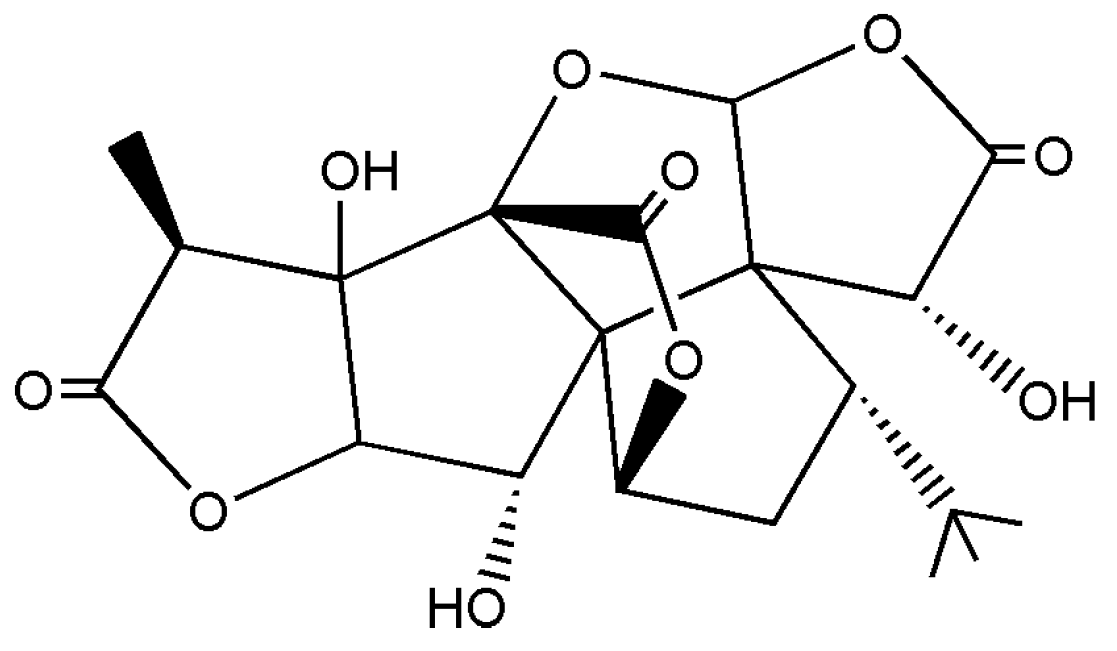
2. Experimental
2.1. Chemicals and Reagents
2.2. Animals
2.3. Instrumentation and LC-MS/MS Conditions
2.3.1. Chromatographic Conditions
2.3.2. Mass Spectrometry Conditions
2.4. Plasma Sample Preparation
2.5. Preparation of Stock and Sample Solutions
2.6. Method Validation
2.6.1. Specificity
2.6.2. Linearity and Lower Limit of Quantitation
2.6.3. Accuracy and Precision
2.6.4. Extraction Recovery and Matrix Effect
2.6.5. Stability
2.6.6. Dilution Effect
2.7. Pharmacokinetic Study
2.8. Statistical Analysis
3. Results and Discussion
3.1. MS/MS Optimization
3.2. Sample Preparation
3.3. Method Validation
3.3.1. Specificity
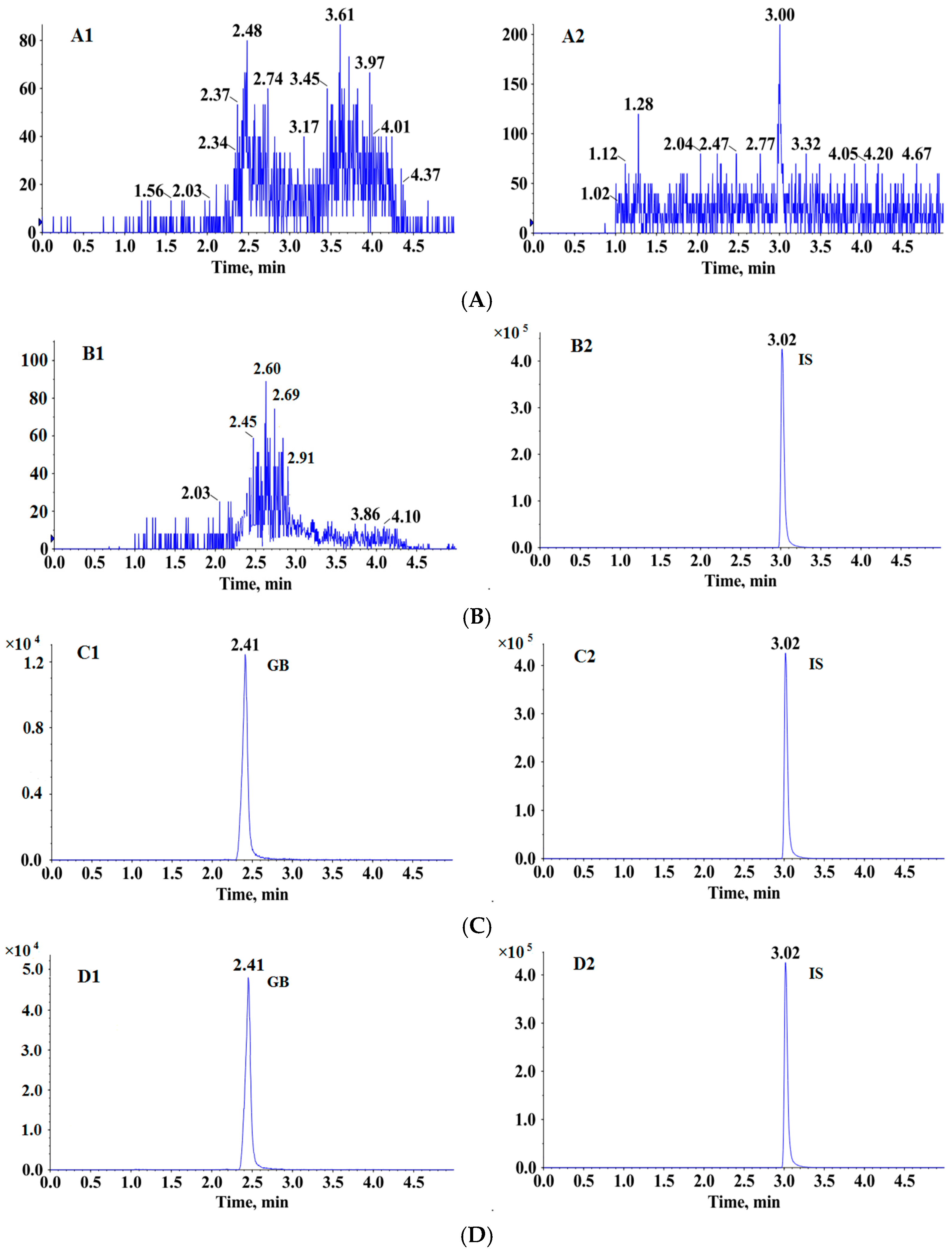
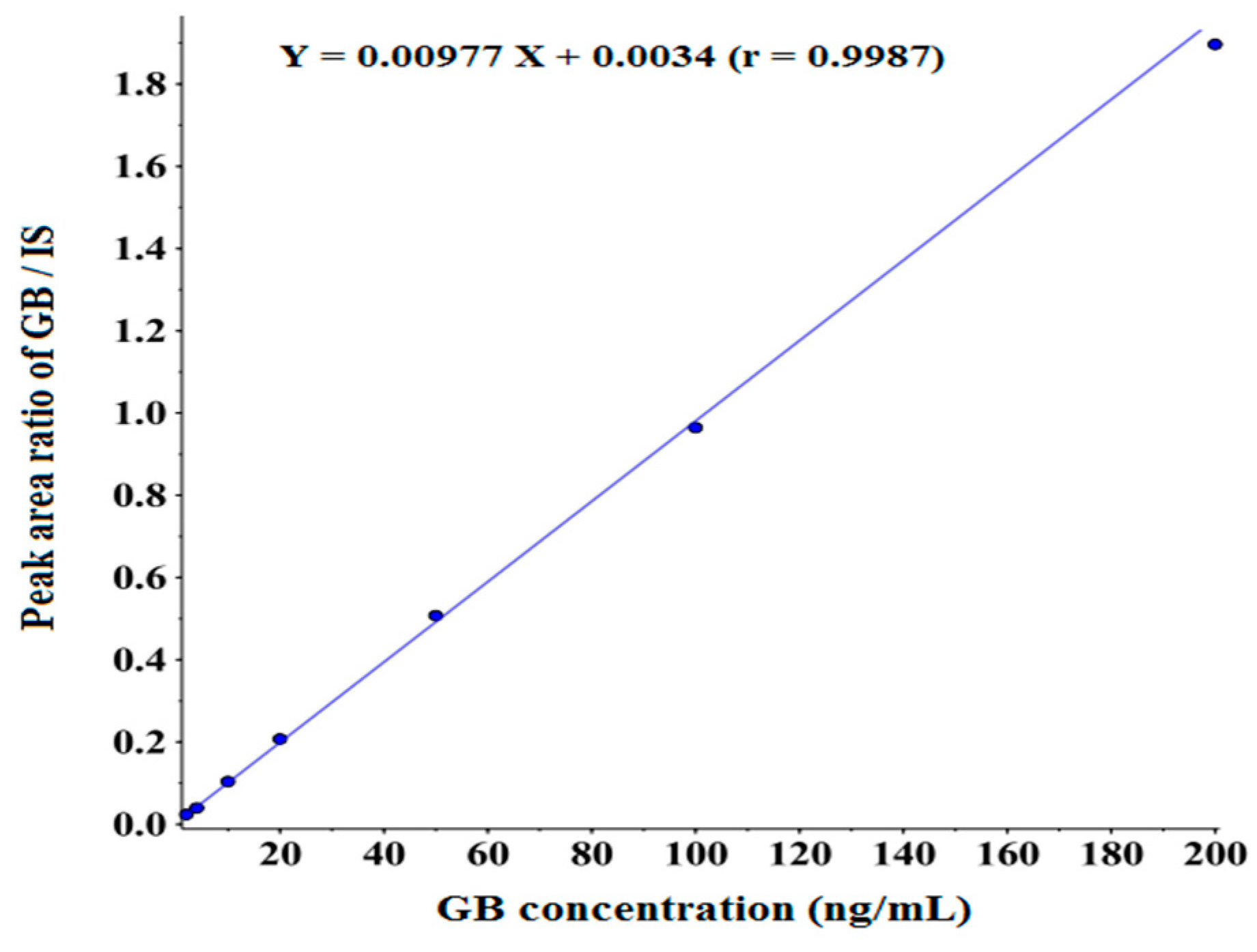
3.3.2. Linearity and LLOQ
3.3.3. Accuracy and Precision
| Concentration (ng/mL) | Intra-Day (n = 6) | Inter-Day (n = 18) | ||||
|---|---|---|---|---|---|---|
| Mean (95% Cl) (ng/mL) | RSD (%) | Accuracy (%) | Mean (95% Cl) (ng/mL) | RSD (%) | Accuracy (%) | |
| 2 (LLOQ) | 1.99 (1.93, 2.05) | 3.9 | 99.3 | |||
| 5 (QCL) | 5.17 (4.77, 5.57) | 9.4 | 103.4 | 4.73 (4.54, 4.92) | 8.6 | 94.6 |
| 80 (QCM) | 90.15 (87.56, 92.74) | 3.6 | 112.7 | 82.79 (79.56, 86.02) | 8.4 | 103.5 |
| 160 (QCH) | 173.7 (171.2, 176.1) | 1.8 | 108.5 | 165.1 (161.6, 168.5) | 4.6 | 103.2 |
3.3.4. Matrix Effect and Recovery
| Concentration (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | |
| 5 (QCL) | 82.09 ± 3.05 | 3.7 | 105.7 ± 2.51 | 2.4 |
| 80 (QCM) | 84.16 ± 3.24 | 2.1 | 99.47 ± 3.37 | 3.4 |
| 160 (QCH) | 81.16 ± 1.65 | 2.0 | 99.70 ± 2.07 | 2.1 |
3.3.5. Stability
| Sample Condition | Concentration (ng/mL) | Mean ± SD (ng/mL) | RSD (%) | Accuracy (%) |
|---|---|---|---|---|
| bench-top stability | 5 | 0.53 ± 1.06 | / | 11.6 |
| 160 | 15.08 ± 1.23 | 8.1 | 9.43 | |
| post-preparative stability | 5 | 5.23 ± 0.48 | 9.1 | 104.6 |
| 160 | 149.8 ± 6.29 | 4.2 | 93.63 | |
| freeze –thaw stability | 5 | 5.19 ± 0.50 | 9.6 | 103.9 |
| 160 | 163.8 ± 11.03 | 6.7 | 102.3 | |
| long-term stability | 5 | 4.69 ± 0.61 | 13.1 | 93.80 |
| 160 | 151.5 ± 5.92 | 3.9 | 94.69 |
3.3.6. Dilution Effect
| Concentration (ng/mL) | Mean ± SD (ng/mL) | RSD (%) | Accuracy (%) |
|---|---|---|---|
| 400 | 427.5 ± 32.12 | 7.5 | 106.9 |
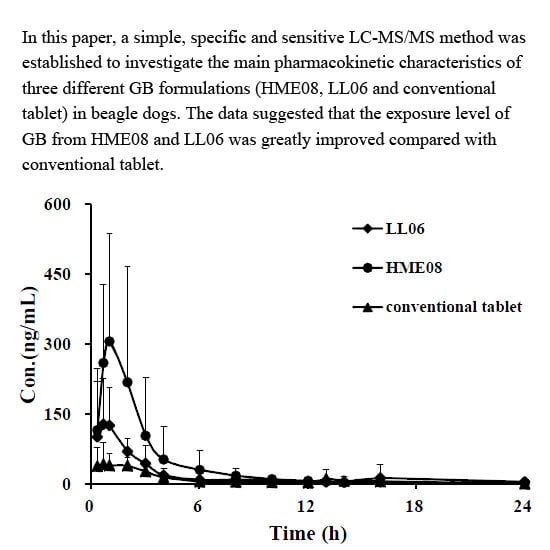
3.4. Pharmacokinetic Study
| Parameters | Conventional Tablet | HME08 | LL06 |
|---|---|---|---|
| AUC0-t (µg/L·h) | 236.2 ± 81.14 | 606.7 ± 125.03 *,# | 419.1 ± 55.58 * |
| AUC0-∞ (µg/L·h) | 246.8 ± 92.08 | 672.0 ± 59.89 *,# | 438.9 ± 71.14 * |
| MRT0-t (h) | 5.65 ± 3.42 | 4.18 ± 1.55 | 3.53 ± 1.77 |
| MRT0-∞ (h) | 6.58 ± 3.54 | 8.75 ± 7.63 | 4.30 ± 2.38 |
| t1/2z (h) | 4.45 ± 3.04 | 4.14 ± 1.81 | 3.14 ± 1.80 |
| Tmax (h) | 3.73 ± 1.21 | 0.80 ± 0.18 | 1.20 ± 1.04 |
| CLz/F (L/h/kg) | 90.94 ± 33.97 | 29.96 ± 2.70 | 46.68 ± 8.65 |
| Vz/F (L/kg) | 560.2 ± 405.4 | 405.7 ± 398.5 | 216.1 ± 132.4 |
| Cmax (µg/L) | 66.64 ± 29.30 | 309.2 ± 106.0 * | 192.4 ± 84.22 * |
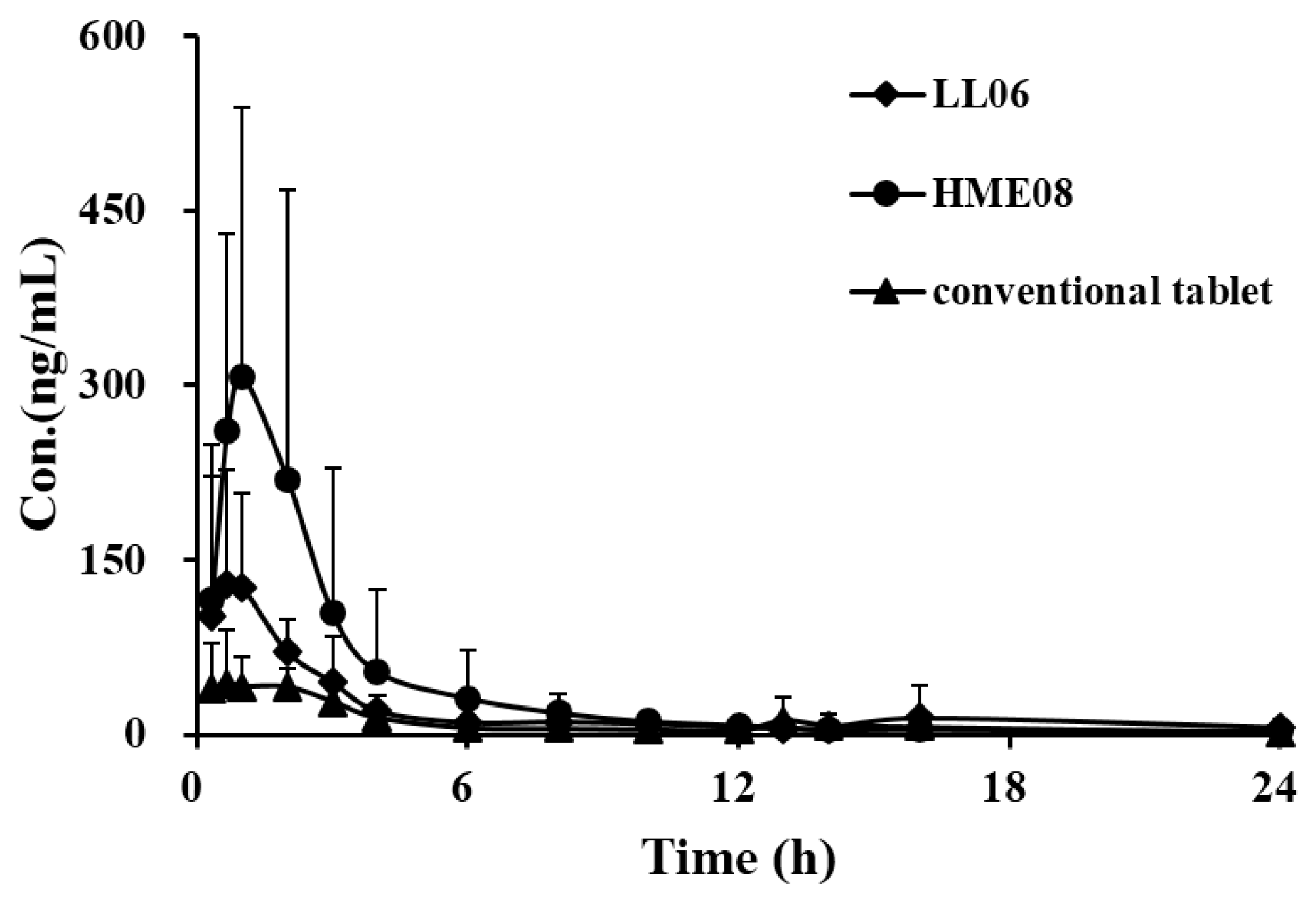
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chan, P.C.; Xia, Q.; Fu, P.P. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Woelkart, K.; Feizlmayr, E.; Dittrich, P.; Beubler, E.; Pinl, F.; Suter, A.; Bauer, R. Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba, L. preparations in humans. Phytother. Res. 2010, 24, 445–450. [Google Scholar] [CrossRef] [PubMed]
- DeFeudis, F.V. A brief history of EGb761 and its therapeutic uses. Pharmacopsychiatry 2003, 36, S2–S7. [Google Scholar] [PubMed]
- Wang, L.; Bai, Y.; Wang, B.; Cui, H.; Wu, H.; Lv, J.R.; Mei, Y.; Zhang, J.S.; Liu, S.; Qi, L.W.; et al. Suppression of experimental abdominal aortic aneurysms in the mice by treatment with Ginkgo biloba extract (EGb 761). J. Ethnopharmacol. 2013, 150, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Maclennan, K.; Darlington, C.L. The neuroprotective properties of the Ginkgo biloba leaf: A review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 1996, 50, 131–139. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, Q.; Song, Y. Improvement of dissolution rate of Ginkgo biloba tablets by using solid dispersion. Chin. New Drugs J. 2004, 13, 521–524. [Google Scholar]
- Tang, J.; Sun, J.; Cui, F.; Zhang, T.; Liu, X.; He, Z. Self-emulsifying drug delivery systems for improving oral absorption of Ginkgo Biloba extracts. Drug Deliv. 2008, 45, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hu, Z.; Zhu, J.; Chen, J. Study on pharmacokinetics of self-microemulsifying drug delivery system of Ginkgo Biloba extract 50. Tradit. Chin. Drug Res. Clin. Pharmacol. 2011, 22, 542–545. [Google Scholar]
- Xiong, Y.; Liu, Q.; Lai, L.; Chen, J. Preparation of the oral self-microemulsifying drug delivery system of GBE50. Acta Pharm Sin. 2009, 44, 803–808. [Google Scholar]
- Tang, J.; Sun, J.; Cui, F.; He, Z. Preparation of self-emulsifying drug delivery systems of Ginkgo biloba Extracts and in Vitro Dissolution Studies. Asian J. Tradit. Med. 2006, 1, 1–4. [Google Scholar]
- Chen, Z.; Sun, J.; Liu, D.; Xiao, Y.; Cai, B. Pharmacokinetics of Ginkgo biloba extract phospholipid complex in rats in vivo. Chin. Tradit. Pat. Med. 2010, 32, 2067–2070. [Google Scholar]
- Chen, Z.P.; Sun, J.; Chen, H.X.; Xiao, Y.Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgobiloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Majumdar, S.; Kumar Battu, S.; Srirangam, R.; Upadhye, S.B. Applications of hot-melt extrusion for drug delivery. Expert. Opin. Drug Deliv. 2008, 5, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Miyagawa, Y.; Okabe, T.; Miyajima, M.; Sunada, H. Dissolution mechanism of diclofenac sodium from wax matrix granules. J. Pharm. Sci. 1997, 86, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, Y.; Sato, H.; Okabe, T.; Nishiyama, T.; Miyajima, M.; Sunada, H. In vivo performance of wax matrix granules prepared by a twin-screw compounding extruder. Drug Dev. Ind. Pharm. 1999, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Albanez, R.; Nitz, M.; Taranto, O.P. Influence of the type of enteric coating suspension, coating layer and process conditions on dissolution profile and stability of coated pellets of diclofenac sodium. Powder Technol. 2015, 269, 185–192. [Google Scholar] [CrossRef]
- Sheahan, T.; Briens, L. Passive acoustic emission monitoring of pellet coat thickness in a fluidized bed. Powder Technol. 2015, 286, 172–180. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, L.; Chai, C.; Qian, X.C.; Li, W.; Li, J.S.; Di, L.Q.; Cai, B.C. Effects of food and gender on the pharmacokinetics of ginkgolides, A.; B, C and bilobalide in rats after oral dosing with ginkgo terpene lactones extract. J. Pharm. Biomed. 2014, 100, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ding, C.; Ge, Q.; Zhou, Z.; Zhi, X. Simultaneous determination of ginkgolides A, B, C and bilobalide in plasma by LC-MS/MS and its application to the pharmacokinetic study of Ginkgo biloba extract in rats. J. Chromatogr. B 2008, 864, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, G.J.; Li, H.; Huang, M.W.; Xie, H.T.; Huang, C.R.; Sun, J.G.; Lv, T. Sensitive and selective liquid chromatography—electrospray ionization mass spectrometry analysis of ginkgolide B in dog plasma. J. Pharm. Biomed. 2006, 40, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry, Bioanalytical Method Validation; US Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Hampton, VA, USA, 2001.
- Li, X.J.; Yang, K.; Du, G.; Xu, L.; Lan, K. Understanding the regioselective hydrolysis of ginkgolide B under physiological environment based on generation, detection, identification, and semi-quantification of the hydrolyzed products. Anal. Bioanal. Chem. 2015, 407, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Ndindayino, F.; Vervaet, C.; van den Mooter, G.; Remon, J.P. Bioavailability of hydrochlorothiazide from isomalt-based moulded tablets. Int. J. Pharm. 2002, 246, 199–202. [Google Scholar] [CrossRef]
- Kinoshita, M.; Baba, K.; Nagayasu, A.; Yamabe, K.; Shimooka, T.; Takeichi, Y.; Azuma, M.; Houchi, H.; Minakuchi, K. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J. Pharm Sci. 2002, 91, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Young, C.R.; Koleng, J.J.; McGinity, J.W. Properties of drug-containing spherical pellets produced by a hot-melt extrusion and spheronization process. J. Microencapsul. 2003, 20, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the HME08, LL06 and conventional GB tablet are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Geng, T.; Wang, Q.; Si, H.; Sun, X.; Guo, Q.; Li, Y.; Huang, W.; Ding, G.; Xiao, W. Pharmacokinetics of Ginkgolide B after Oral Administration of Three Different Ginkgolide B Formulations in Beagle Dogs. Molecules 2015, 20, 20031-20041. https://doi.org/10.3390/molecules201119678
Zhao J, Geng T, Wang Q, Si H, Sun X, Guo Q, Li Y, Huang W, Ding G, Xiao W. Pharmacokinetics of Ginkgolide B after Oral Administration of Three Different Ginkgolide B Formulations in Beagle Dogs. Molecules. 2015; 20(11):20031-20041. https://doi.org/10.3390/molecules201119678
Chicago/Turabian StyleZhao, Jie, Ting Geng, Qi Wang, Haihong Si, Xiaoping Sun, Qingming Guo, Yanjing Li, Wenzhe Huang, Gang Ding, and Wei Xiao. 2015. "Pharmacokinetics of Ginkgolide B after Oral Administration of Three Different Ginkgolide B Formulations in Beagle Dogs" Molecules 20, no. 11: 20031-20041. https://doi.org/10.3390/molecules201119678