A Simple, Effective, Green Method for the Regioselective 3-Acylation of Unprotected Indoles
Abstract
:1. Introduction
2. Results and Discussion

| 0 | Metal Triflate | Isolated Yield (%) | Selective Position (1-/2-/3-) |
|---|---|---|---|
| 1 | Cu(OTf)2 | 74 | 5/0/95 |
| 2 | Y(OTf)3 | 84 | 3/0/97 |
| 3 | In(OTf)3 | 80 | 4/0/96 |
| 4 | Bi(OTf)3 | 70 | 9/0/91 |
| 5 | La(OTf)3 | 81 | 4/0/96 |
| 6 | Ce(OTf)3 | 79 | 4/0/96 |
| 7 | Pr(OTf)3 | 70 | 4/0/96 |
| 8 | Nd(OTf)3 | 74 | 5/0/95 |
| 9 | Eu(OTf)3 | 73 | 4/0/96 |
| 10 | Gd(OTf)3 | 76 | 5/0/95 |
| 11 | Tb(OTf)3 | 76 | 4/0/96 |
| 12 | Dy(OTf)3 | 78 | 4/0/96 |
| 13 | Ho(OTf)3 | 75 | 4/0/96 |
| 14 | Tm(OTf)3 | 80 | 4/0/96 |
| Entry | Solvent | Yield b (%) | Selective Position (1-/2-/3-) c |
|---|---|---|---|
| 1 | n-hexane | 20 | 5/0/95 |
| 2 | cyclopentyl methyl ether | 50 | 9/0/91 |
| 3 | tert-butyl methyl ether | 27 | 0/0/100 |
| 4 | dioxane | 63 | 3/0/97 |
| 5 | tetrahydrofuran | 50 | 4/0/96 |
| 6 | ethyl acetate | 63 | 3/0/97 |
| 7 | chloroform | 34 | 10/0/90 |
| 8 | dichloromethane | 22 | 0/0/100 |
| 9 | acetone | 44 | 37/0/63 |
| 10 | acetonitrile | 68 | 2/0/100 |
| 11 | methanol | 0 | - |
| 12 | dimethyl carbonate | 57 | 3/0/97 |
| 13 | [BMI]BF4 | 92 | 0/0/100 |
| 14 | [BMI]Cl | 66 | 5/0/95 |
| 15 | [BMI]PF6 | 70 | 3/0/97 |
| 16 | [BMI]SCN | 43 | 22/0/78 |
| 17 | [BPy]OTf | 68 | 6/0/94 |
| 18 | [EMI]Cl | 74 | 4/0/96 |
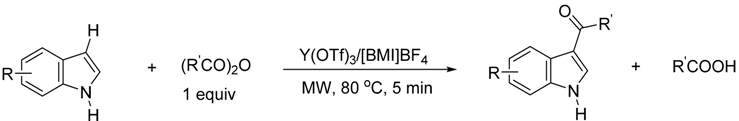
| Entry | Substrate | R′ | Selective Position in Parenthesis b(1-/2-/3-) | Isolated Yield (%) of 3-Substituted Derivative c |
|---|---|---|---|---|
| 1 |  | CH3 | 0/0/100 | 88 d |
| 2 | C2H5 | 0/0/100 | 92 | |
| 3 | C3H7 | 3/0/97 | 88 e | |
| 4 | i-C3H7 | 2/2/96 | 91 | |
| 5 | t-C4H9 | 1/4/95 | 89 | |
| 6 | C6H5 | 5/3/92 | 78 f | |
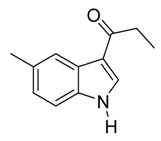
| 7 | 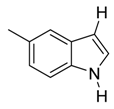 | CH3 | 6/0/94 | 79 g |
| 8 | C2H5 | 6/1/93 | 80 | |
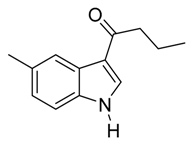
| 9 | C3H7 | 7/2/91 | 79 | |
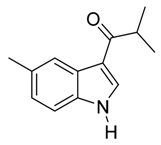
| 10 | i-C3H7 | 1/8/91 | 80 | |
| 11 | t-C4H9 | 1/6/93 | 83 | |
| 12 | C6H5 | 3/4/93 | 83 | |
| 13 |  | CH3 | 8/0/92 | 79 g |
| 14 | C2H5 | 6/2/92 | 78 | |
| 15 | C3H7 | 6/2/92 | 80 | |
| 16 | i-C3H7 | 2/7/91 | 78 | |
| 17 | t-C4H9 | 1/4/95 | 79 | |
| 18 | C6H5 | 7/4/89 | 78 | |
| 19 |  | CH3 | 4/2/94 | 83 h |
| 20 | C2H5 | 4/1/95 | 85 g | |
| 21 | C3H7 | 5/2/93 | 78 | |
| 22 | i-C3H7 | 2/5/93 | 80 | |
| 23 | t-C4H9 | 1/4/95 | 80 g | |
| 24 | C6H5 | not determined i | 75 | |
| 25 |  | CH3 | 3/1/96 | 79 h |
| 26 | C2H5 | 2/2/96 | 82 | |
| 27 | C3H7 | 3/2/95 | 80 | |
| 27 | i-C3H7 | 2/7/91 | 79 | |
| 29 | t-C4H9 | 6/15/79 | 65 g | |
| 30 | C6H5 | 1/9/90 | 77 | |
| 31 | 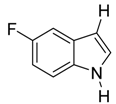 | C2H5 | 3/3/94 | 80 |
| 32 | t-C4H9 | 3/39/58 | 45 | |
| 33 | 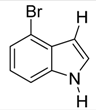 | C2H5 | 10/0/90 | 72 |
| 34 | t-C4H9 | 4/4/92 | 82 |
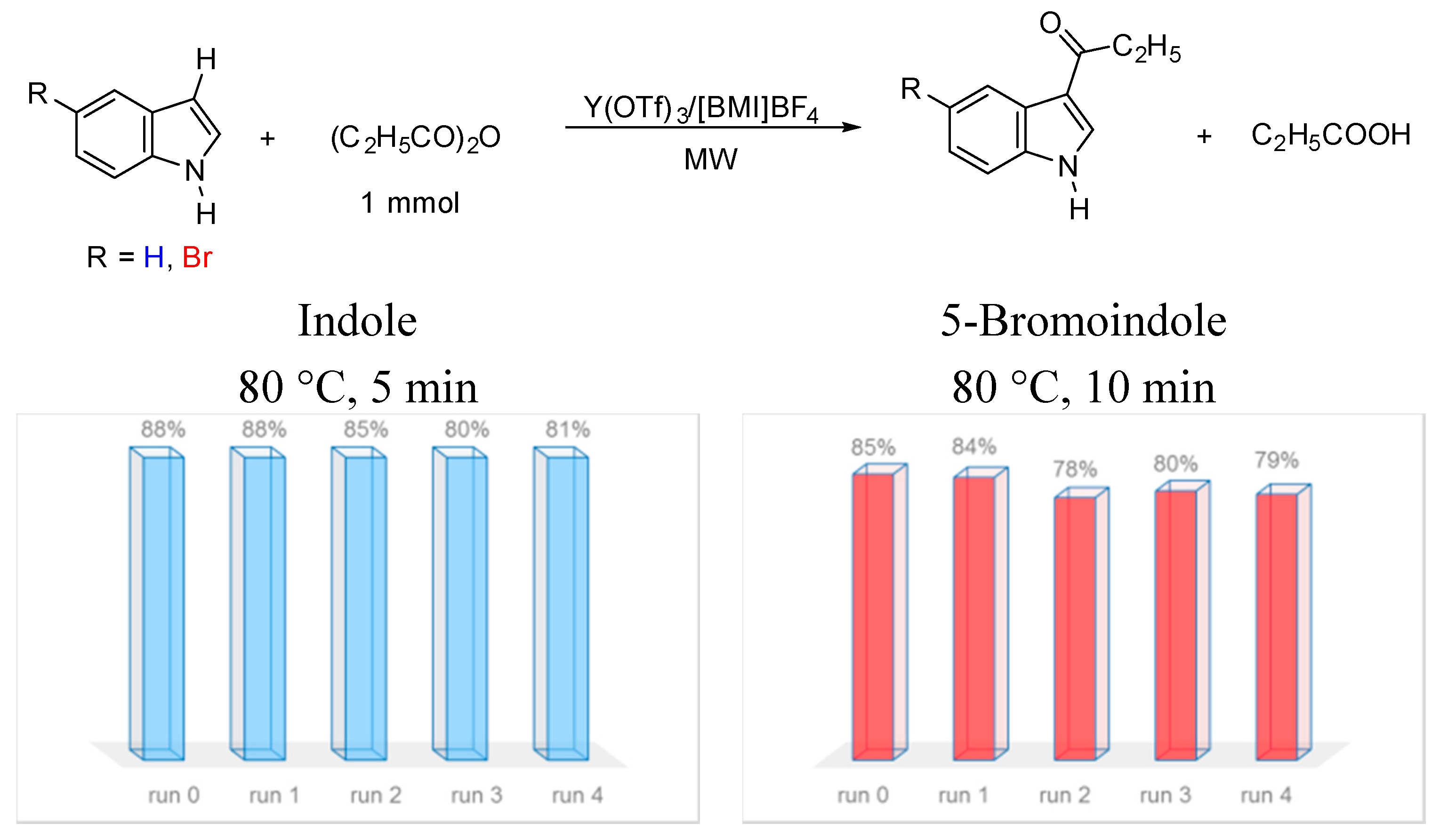
3. Experimental Section
3.1. Chemicals and Supplies
3.2. Instruments
3.3. Acylation Procedure
3.4. Recovery and Reuse of the Catalytic System Y(OTf)3/[BMI]BF4
3.5. Compounds
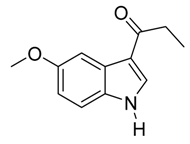
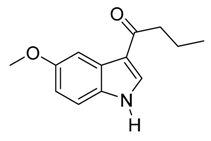

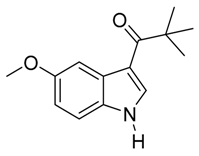

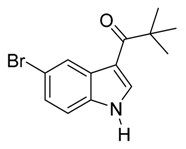
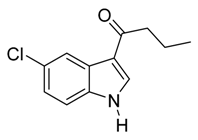
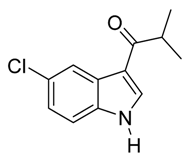
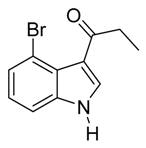
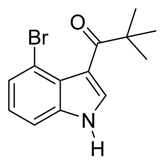
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvarez-Builla, J.; Vaquero, J.J.; Barluenga, J. Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011; Volume 4, pp. 377–532. [Google Scholar]
- Velavan, A.; Sumathi, S.; Balasubramanian, K.K. AlMe3-mediated regio- and chemoselective reactions of indole with carbamoyl electrophiles. Eur. J. Org. Chem. 2013, 3148–3157. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, T.; Xiong, S.; Dong, X.; Zhou, L. Synthesis of 3-acylindoles by visible-light induced intramolecular oxidative cyclization of o-alkynylated N,N-dialkylamines. Org. Lett. 2014, 16, 3264–3267. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Zhang, S.; Shao, Y.; Fan, L.; Xu, H.; Yu, X.; Zhi, X.; Yao, X.; Zhang, R. Synthesis and quantitative structure-activity relationship (QSAR) study of novel N-arylsulfonyl-3-acylindole arylcarbonyl hydrazone derivatives as nematicidal agents. J. Agric. Food. Chem. 2013, 61, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, O.; Neder, A.D.F.; Dias, A.K.B.; Cruz, R.P.A.; Aquino, L.B. Acylation of indole under Friedel-Crafts conditions—An improved method to obtain 3-acylindoles regioselectively. Org. Lett. 2001, 3, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Stalick, W.M.; Wynne, J.H.; Lloyd, C.T.; Jensen, S.D.; Boson, S. 3-Acylindoles via a One-Pot, Regioselective Friedel-Crafts reaction. Synthesis 2004, 2277–2282. [Google Scholar] [CrossRef]
- Lin, H.; Sun, X.W. Highly efficient synthesis of 3-indolyl-substituted phthalides via Friedel-Crafts reactions in water. Tetrahedron Lett. 2008, 49, 5343–5346. [Google Scholar] [CrossRef]
- Metwally, M.A.; Shaaban, S.; Abdel-Wahab, B.F.; El-Hiti, G.A. 3-Acetylindoles: Synthesis, reactions and biological activities. Curr. Org. Chem. 2009, 13, 1475–1496. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Kashyap, M.; Kamble, H. ZrCl4-mediated regio- and chemoselective Friedel-Crafts acylation of indole. J. Org. Chem. 2011, 76, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Li, T.; Yang, Y.; Zhang, H.; Lan, X.; Li, F.; Han, J.; Ma, Z.; Chen, Q.; Chen, G. Microwave-assisted Friedel-Crafts acylation of indole with acetic anhydride over tungstophosphoric acid modified Hβ zeolite. Catal. Commun. 2012, 29, 114–117. [Google Scholar] [CrossRef]
- Zhang, L.R.; Yi, F.P.; Zou, J.Z.; Zhang, X.; Wang, Z. Regioselective Friedel-Crafts acylation of indoles catalysed by zinc oxide in an ionic liquid. J. Chem. Res. 2012, 36, 600–602. [Google Scholar] [CrossRef]
- Lai, Q.Y.; Liao, R.S.; Wu, S.Y.; Zhang, J.X.; Duan, X.H. A novel microwave-irradiated solvent-free 3-acylation of indoles on alumina. New J. Chem. 2013, 37, 4069–4076. [Google Scholar] [CrossRef]
- Chatterjee, A.; Biswas, K.M. Acylation of indoles by Duff reaction and Vilsmeier-Haack formylation and conformation of N-formylindoles. J. Org. Chem. 1973, 38, 4002–4004. [Google Scholar] [CrossRef]
- Tang, R.Y.; Guo, X.K.; Xiang, J.N.; Li, J.H. Palladium-catalyzed synthesis of 3-acylated indoles involving oxidative cross-coupling of indoles with alpha-amino carbonyl compounds. J. Org. Chem. 2013, 78, 11163–11171. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, P.; Wang, L. Copper-promoted decarboxylative direct C3-acylation of N-substituted indoles with alpha-oxocarboxylic acids. Chem. Commun. 2013, 49, 2368–2370. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.J.; Liu, J.Y.; Zhang, L.Z.; Xiong, Y.; Wang, R. Synthesis of 3-acylindoles via decarboxylative cross-coupling reaction of free (N-H) indoles with α-oxocarboxylic acids. Chin. Chem. Lett. 2014, 25, 90–92. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, H.; Yan, J.; Chi, H.; Chen, X.; Zhang, Z. Copper-catalyzed decarboxylative C3-acylation of free (N-H) indoles with alpha-oxocarboxylic acids. Org. Biomol. Chem. 2014, 12, 1721–1724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.S.; Wang, G.W. Synthesis of 3-acylindoles by palladium-catalyzed acylation of free (N-H) indoles with nitriles. Org. Lett. 2013, 15, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes; Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ketcha, D.M.; Gribble, G.W. A convenient synthesis of 3-acylindoles via Friedel-Crafts acylation of 1-(pheny1sulfonyl)indole. A new route to pyridocarbazole-5,11-quinones and Ellipticine. J. Org. Chem. 1985, 50, 5451–5457. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Olah, G.A. Friedel-Crafts Chemistry; John Wiley and Sons: New York, NY, USA, 1973. [Google Scholar]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.L. Rare-earth metal triflates in organic synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Mathew, T.; Olah, G.A. Gallium(III) triflate: An efficient and a sustainable Lewis acid catalyst for organic synthetic transformations. Acc. Chem. Res. 2012, 45, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, A.; Selhorst, R.; Turnbull, K. Metal triflate-catalyzed Friedel-Crafts acetylation of 3-phenylsydnone. Synth. Commun. 2013, 43, 1626–1632. [Google Scholar] [CrossRef]
- Perrier, A.; Keller, M.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Efficient and recyclable rare earth-based catalysts for Friedel-Crafts acylations under microwave heating: Dendrimers show the way. Green Chem. 2013, 15, 2075–2080. [Google Scholar] [CrossRef]
- Rani, A.; Khatri, C.; Hada, R. Fly ash supported scandium triflate as an active recyclable solid acid catalyst for Friedel-Crafts acylation reaction. Fuel Process. Technol. 2013, 116, 366–373. [Google Scholar] [CrossRef]
- Tran, P.H.; Hansen, P.E.; Pham, T.T.; Huynh, V.T.; Huynh, V.H.; Thi Tran, T.D.; Huynh, T.V.; Le, T.N. Microwave-assisted facile and rapid Friedel-Crafts benzoylation of arenes catalyzed by bismuth trifluoromethanesulfonate. Synth. Commun. 2014, 44, 2921–2929. [Google Scholar] [CrossRef]
- Nagarajan, R.; Perumal, P.T. InCl3 and In(OTf)3 catalyzed reactions: Synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivatives. Tetrahedron 2002, 58, 1229–1232. [Google Scholar] [CrossRef]
- Luo, S.; Zhu, L.; Talukdar, A.; Zhang, G.; Mi, X.; Cheng, J.P.; Wang, P.G. Recent advances in rare earth-metal triflate catalyzed organic synthesis in green media. Mini-Rev. Org. Chem. 2005, 2, 177–202. [Google Scholar] [CrossRef]
- Ross, J.; Xiao, J. Friedel-Crafts acylation reactions using metal triflates in ionic liquid. Green Chem. 2002, 4, 129–133. [Google Scholar] [CrossRef]
- Gmouh, S.; Yang, H.; Vaultier, M. Activation of bismuth(III) derivatives in ionic liquids: Novel and recyclable catalytic systems for Friedel-Crafts acylation of aromatic compounds. Org. Lett. 2003, 5, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, W.; Lin, J.; Chen, M.; Li, J. Friedel-Crafts acylation of ferrocene catalyzed by immobilized ytterbium(III) triflate in ionic liquid. Synth. Commun. 2005, 35, 1929–1937. [Google Scholar] [CrossRef]
- Goodrich, P.; Hardacre, C.; Mehdi, H.; Nancarrow, P.; Rooney, D.W.; Thompson, J.M. Kinetic study of the metal triflate catalyzed benzoylation of anisole in an ionic liquid. Ind. Eng. Chem. Res. 2006, 45, 6640–6647. [Google Scholar] [CrossRef]
- Zayed, F.; Greiner, L.; Schulz, P.S.; Lapkin, A.; Leitner, W. Continuous catalytic Friedel-Crafts acylation in the biphasic medium of an ionic liquid and supercritical carbon dioxide. Chem. Commun. 2008, 79–81. [Google Scholar] [CrossRef]
- Tran, P.H.; Duus, F.; Le, T.N. Friedel-Crafts acylation using bismuth triflate in [BMI][PF6]. Tetrahedron Lett. 2012, 53, 222–224. [Google Scholar] [CrossRef]
- Tran, P.H.; Do, N.B. L.; Le, T.N. Improvement of the Friedel-Crafts benzoylation by using bismuth trifluoromethanesulfonate in 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid under microwave irradiation. Tetrahedron Lett. 2014, 55, 205–208. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Wang, Y.; Onozawa, S.-Y.; Kunioka, M. Ring-opening polymerization of caprolactone with yttrium triflate. Green Chem. 2003, 5, 571–574. [Google Scholar] [CrossRef]
- De, S.K. Yttrium triflate as an efficient and useful catalyst for chemoselective protection of carbonyl compounds. Tetrahedron Lett. 2004, 45, 2339–2341. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. A mild and efficient one-step synthesis of quinolines. Tetrahedron Lett. 2005, 46, 1647–1649. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Mishra, A.K.; Saha, S.; Ray, B. Synthesis and characterization of stereocontrolled poly(N-isopropylacrylamide) hydrogel prepared in the presence of Y(OTf)3 Lewis acid. Langmuir 2010, 26, 6775–6782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lin, L.; Wang, W.; Ji, J.; Liu, X.; Feng, X. Highly enantioselective Michael addition of malonates to beta,gamma-unsaturated alpha-ketoesters catalyzed by chiral N,N′-dioxide-Yttrium(III) complexes with convenient procedure. Chem. Commun. 2010, 46, 3601–3603. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.; Enaohwo, O.; Guy, S. Yttrium triflate-catalyzed efficient chemoselective S-benzylation of indoline-2-thiones using benzyl alcohols. Tetrahedron Lett. 2011, 52, 684–687. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.; Zhang, J. Lewis acid-catalyzed formal [3+2] cycloadditions of N-tosyl aziridines with electron-rich alkenes via selective carbon-carbon bond cleavage. Chem. Commun. 2011, 47, 5049–5051. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Li, B.J.; Shi, Z.J. Lewis acid catalyzed benzylic C-H bond functionalization of azaarenes: Addition to enones. Org. Lett. 2011, 13, 1706–1709. [Google Scholar]
- Bittner, B.; Pełech, R.; Janus, E.; Milchert, E. Synthesis of 2-propanoyl-5-norbornene in pyridinium ionic liquids catalyzed by yttrium salts. Catal. Lett. 2012, 142, 332–337. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chanda, T.; Nandi, G.C.; Koley, S.; Janaki Ramulu, B.; Pandey, S.K.; Singh, M.S. Y(OTf)3 catalyzed substitution dependent oxidative C(sp3)–C(sp3) cleavage and regioselective dehydration of β-allyl-β-hydroxydithioesters: Alternate route to α,β-unsaturated ketones and functionalized dienes. Tetrahedron 2013, 69, 8899–8903. [Google Scholar] [CrossRef]
- Berardi, S.; Conte, V.; Fiorani, G.; Floris, B.; Galloni, P. Improvement of ferrocene acylation. Conventional vs. microwave heating for scandium-catalyzed reaction in alkylmethylimidazolium-based ionic liquids. J. Organomet. Chem. 2008, 693, 3015–3020. [Google Scholar] [CrossRef]
- Pearson, D.E. Pivalophenones from Friedel-Crafts and Grignard Reactions. J. Am. Chem. Soc. 1950, 72, 4169–4170. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compouns are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, P.H.; Tran, H.N.; Hansen, P.E.; Do, M.H.N.; Le, T.N. A Simple, Effective, Green Method for the Regioselective 3-Acylation of Unprotected Indoles. Molecules 2015, 20, 19605-19619. https://doi.org/10.3390/molecules201019605
Tran PH, Tran HN, Hansen PE, Do MHN, Le TN. A Simple, Effective, Green Method for the Regioselective 3-Acylation of Unprotected Indoles. Molecules. 2015; 20(10):19605-19619. https://doi.org/10.3390/molecules201019605
Chicago/Turabian StyleTran, Phuong Hoang, Hai Ngoc Tran, Poul Erik Hansen, Mai Hoang Ngoc Do, and Thach Ngoc Le. 2015. "A Simple, Effective, Green Method for the Regioselective 3-Acylation of Unprotected Indoles" Molecules 20, no. 10: 19605-19619. https://doi.org/10.3390/molecules201019605








