Enhanced Visible Light Photocatalytic Activity of Br-Doped Bismuth Oxide Formate Nanosheets
Abstract
:1. Introduction
2. Results and Discussion
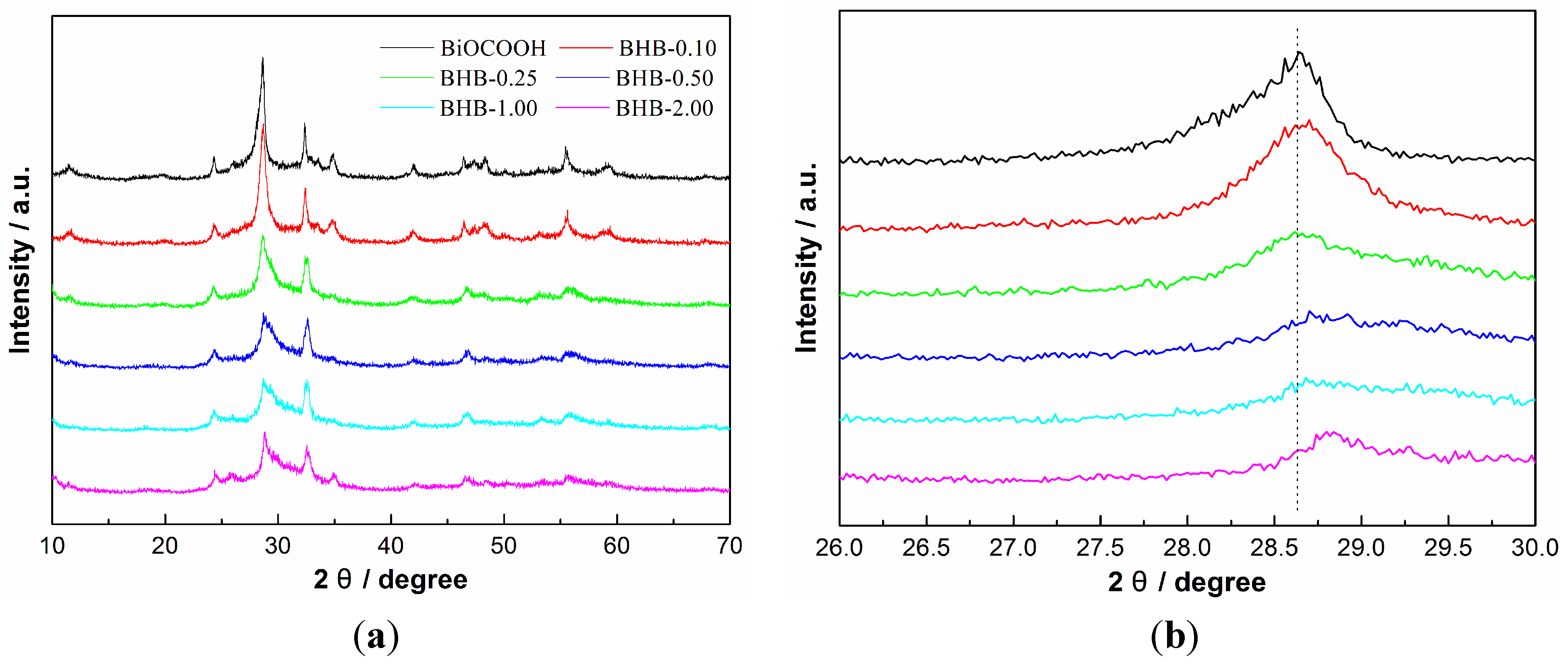
2.1. Phase Structure
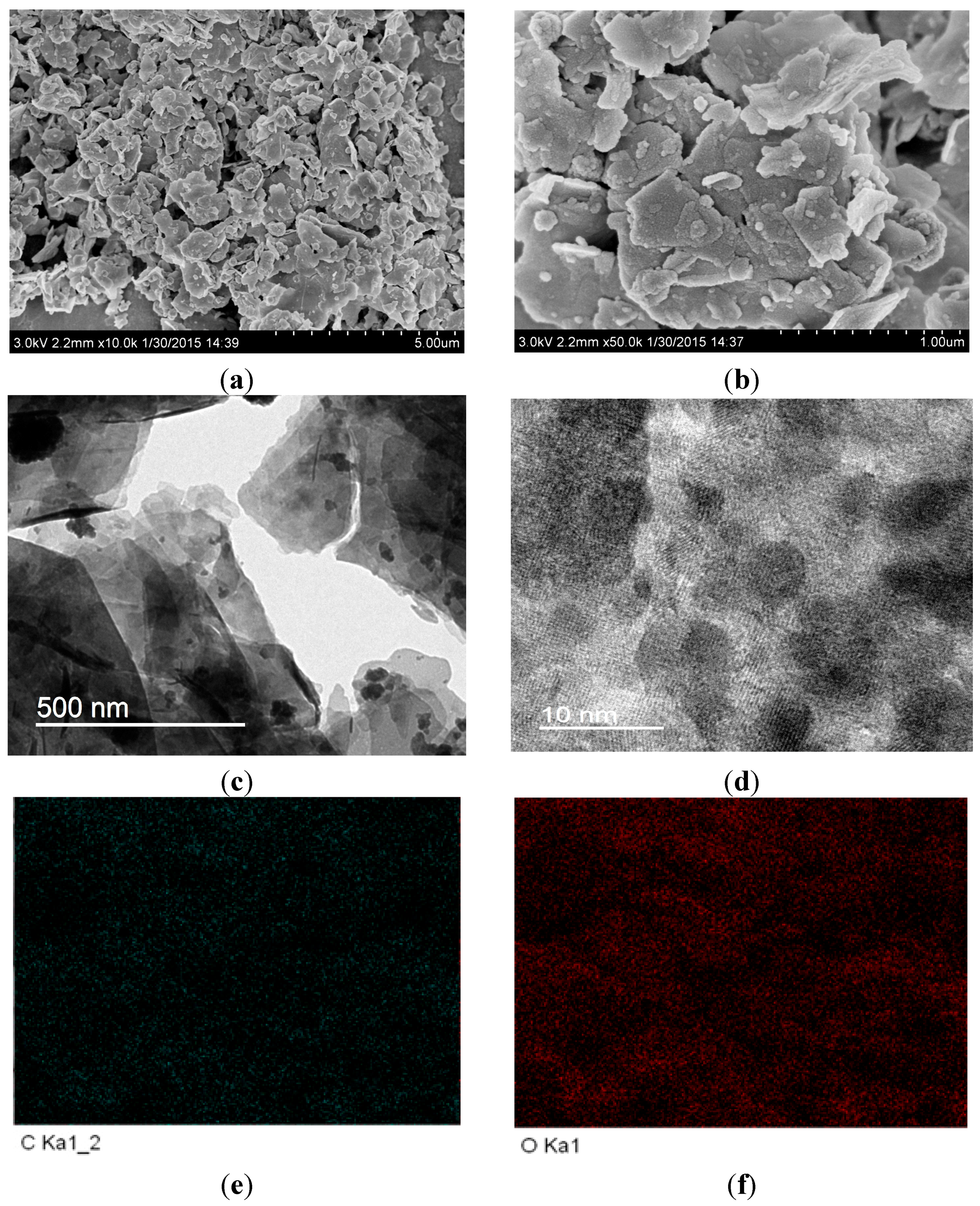
2.2. Morphological Structure

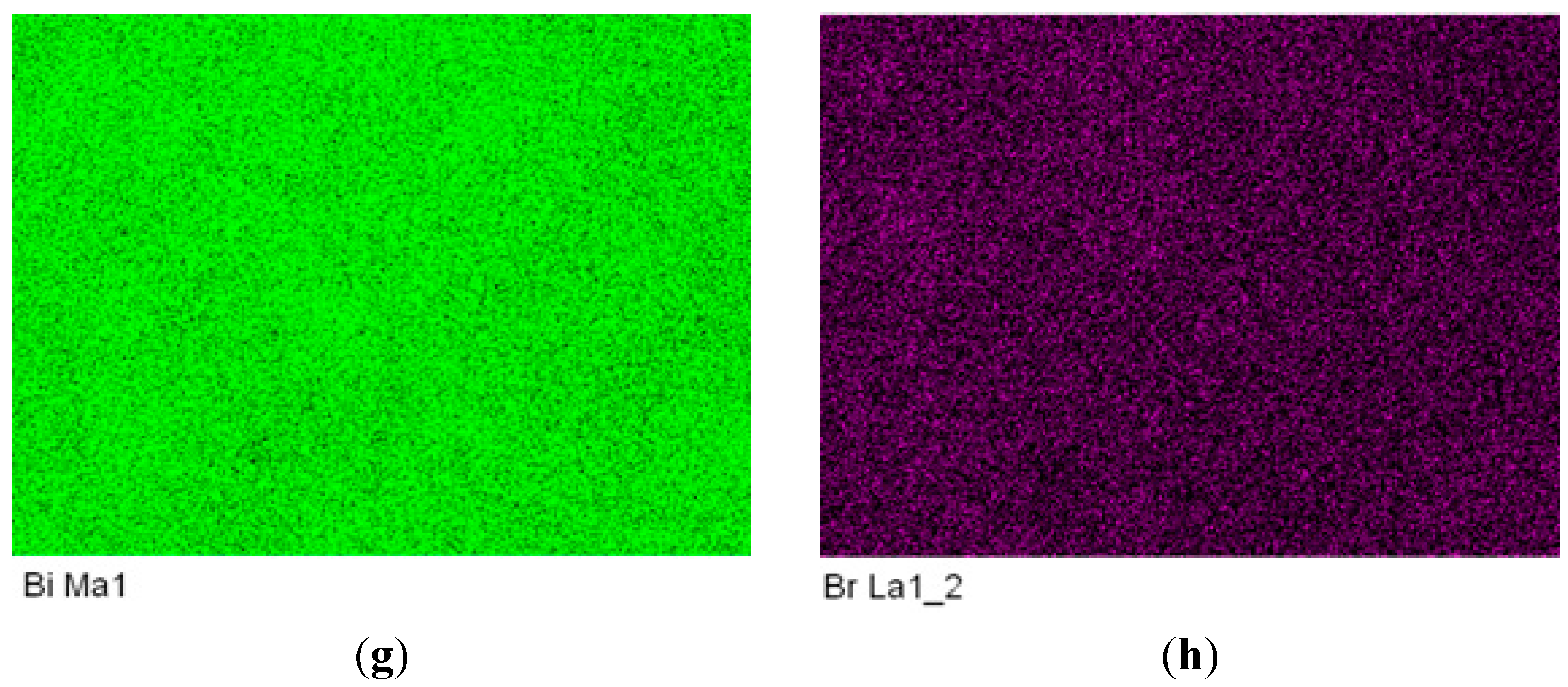
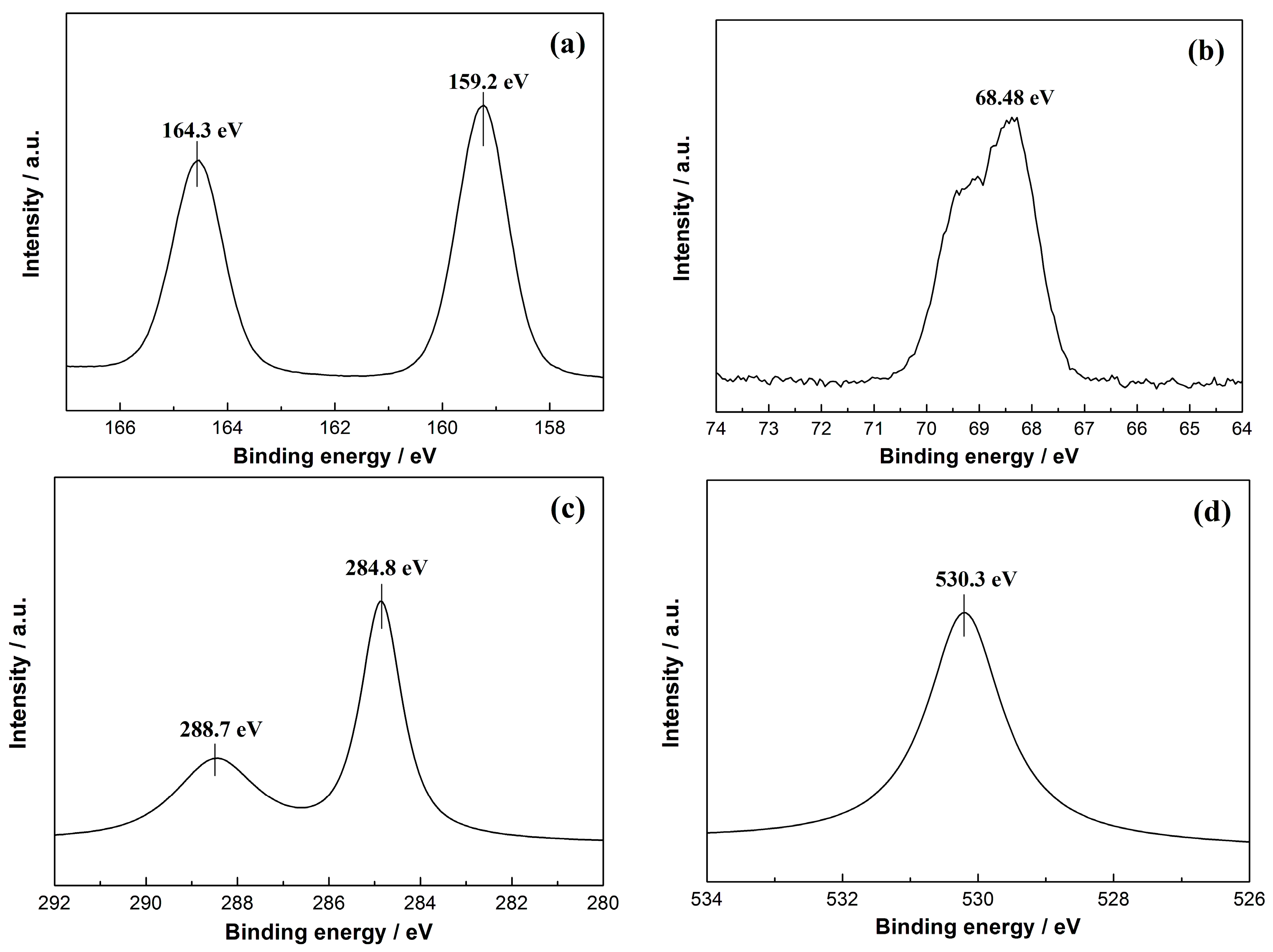
2.3. Local Chemical Structure by XPS
2.4. BET Surface Areas and Pore Structure
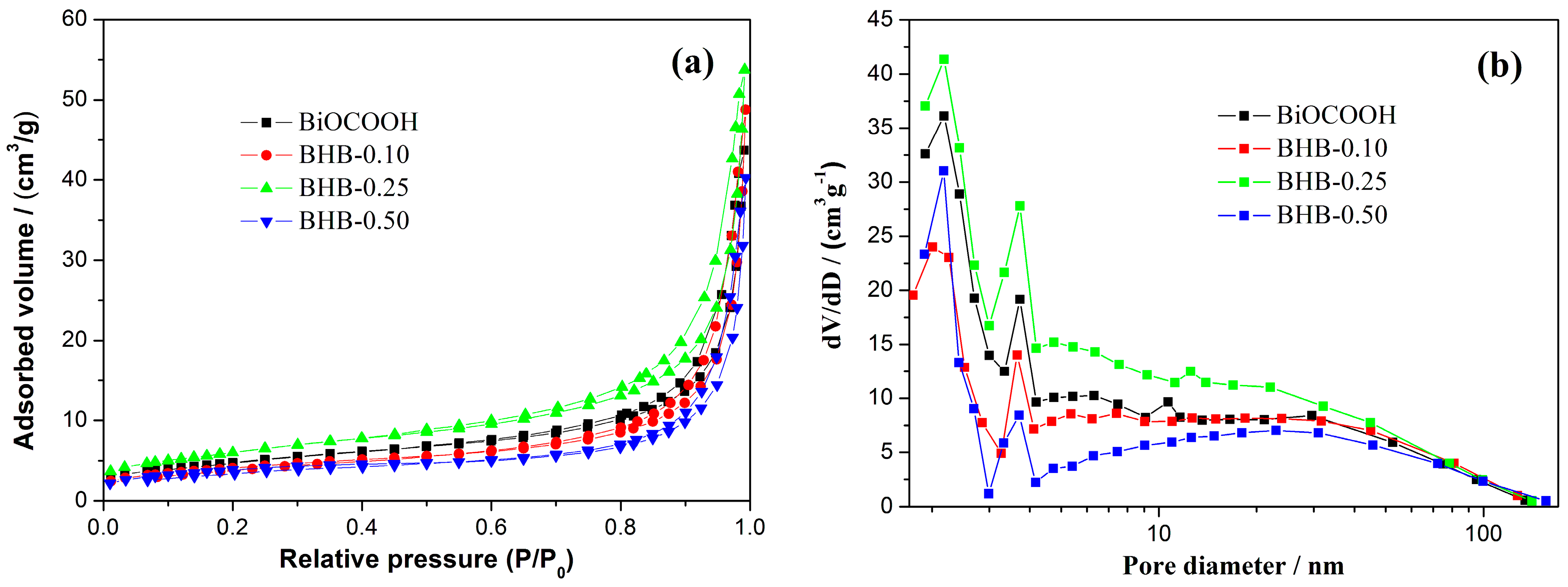
| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Peak Pore Diameter (nm) | NO η (%) |
|---|---|---|---|---|
| BHB | 12.98 | 0.067 | 1.2/2.8 | 0 |
| BHB-0.10 | 14.69 | 0.075 | 1.2/2.8 | 31.4 |
| BHB-0.25 | 21.93 | 0.083 | 1.2/2.8 | 37.6 |
| BHB-0.50 | 13.64 | 0.062 | 1.2/2.8 | 33.5 |
2.5. Light Absorption and Charge Separation



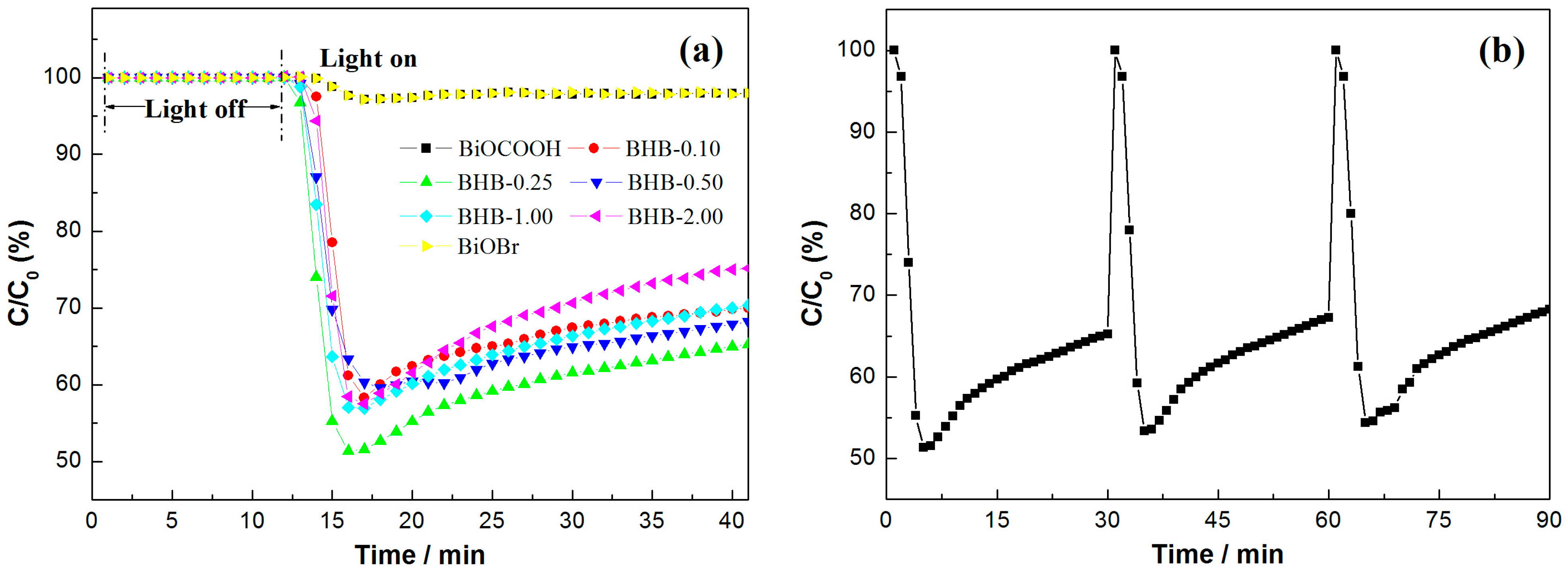
2.6. Visible Light Photocatalytic Activity
3. Experimental Section
3.1. Synthesis of BiOCOOH Nanosheets
3.2. Synthesis of Br-Doped BiOCOOH Nanosheets
3.3. Synthesis of BiOBr
3.4. Characterization
3.5. Evaluation of Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, X.B.; Lu, Z.F.; Zhu, L.W.; Yang, L.; Gu, L.; Cai, L.L.; Chen, J. A new family of sunlight-driven bifunctional photocatalysts based on TiO2 nanoribbon frameworks and bismuth oxohalide nanoplates. Nanoscale 2014, 6, 1434–1444. [Google Scholar] [PubMed]
- Zhang, L.S.; Wang, H.L.; Chen, Z.G.; Wong, P.K.; Liu, J.S. Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Zhang, N.; Ciriminna, R.; Pagliaro, M.; Xu, Y.J. Nanochemistry-derived Bi2WO6 nanostructures: Towards production of sustainable chemicals and fuels induced by visible light. Chem. Soc. Rev. 2014, 43, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Pan, L.; Zhou, J.; Zhang, X.W.; Wang, L. Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: A review on recent progress. Nanoscale 2014, 6, 14044–14063. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; Kuang, Q.; Jin, M.S.; Xie, Z.X.; Zheng, L.X. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Mclaren, A.; Valdes-Solis, T.; Li, G.Q.; Tsang, S.C. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Y.F. Synthesis of square Bi2WO6 nanoplates as high-activity visible-light-driven photocatalysts. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Zhang, L.W.; Xu, T.G.; Zhao, X.; Zhu, Y.F. Controllable Synthesis of Bi2MoO6 and Effect of Morphology and Variation in Local Structure on Photocatalytic Activities. Appl. Catal. B 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Halpin, Y.; Pryce, M.T.; Rau, S.; Dini, D.; Vos, J.G. Recent progress in the development of bimetallic photocatalysts for hydrogen generation. Dalton Trans. 2013, 42, 16243–16254. [Google Scholar] [CrossRef] [PubMed]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between Surface Area and Photocatalytic Activity for Acetaldehyde Decomposition over Bismuth Tungstate Particles with a Hierarchical Structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.Y.; Dong, F.; Zhang, W.; Wu, Z.B. Efficient visible light photocatalytic oxidation of NO in air with band-gap tailored (BiO)2CO3-BiOI solid solutions. Chem. Eng. J. 2014, 255, 650–658. [Google Scholar] [CrossRef]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Generalized One-Pot Synthesis, Characterization, and Photocatalytic Activity of Hierarchical BiOX (X = Cl, Br, I) Nanoplate Microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Huo, Y.N.; Miao, M.; Zhang, Y.; Zhu, J.; Li, H.X. Aerosol-Spraying Preparation of a Mesoporous Hollow Spherical BiFeO3 Visible Photocatalyst with Enhanced Activity and Durability. Chem. Commun. 2011, 47, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.F.; Huo, Y.N.; Zhang, Y.; Zhu, J.; Lu, Y.F. Aerosol-Spay Assisted Assembly of Bi2Ti2O7 Crystals in Uniform Porous Microspheres with Enhanced Photocatalytic Activity. Appl. Catal. B 2009, 91, 247–253. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, F.Q.; Lü, X.J.; Chen, P.; Wan, D.Y.; Xu, F.F. Improved Visible-Light Lhotocatalysis of Nano-Bi2Sn2O7 with Dispersed S-Bands. J. Mater. Chem. 2011, 21, 3872–3876. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, W.D. Anion Exchange Strategy for Construction of Sesame-Biscuit-Like Bi2O2CO3/Bi2MoO6 Nanocomposites with Enhanced Photocatalytic Activity. Appl. Catal. B 2013, 140, 306–316. [Google Scholar] [CrossRef]
- Yao, W.F.; Xu, X.H.; Wang, H.; Zhou, J.T.; Yang, X.N.; Zhang, Y.; Shang, S.X.; Huang, B.B. Photocatalytic Property of Perovskite Bismuth Titanate. Appl. Catal. B 2004, 52, 109–116. [Google Scholar] [CrossRef]
- Xiong, T.; Huang, H.W.; Sun, Y.J.; Dong, F. In-situ synthesis of a C-doped (BiO)2CO3 hierarchical self-assembly effectively promoting visible light photocatalysis. J. Mater. Chem. A 2015, 3, 6118–6127. [Google Scholar] [CrossRef]
- Dong, F.; Lee, S.C.; Wu, Z.B.; Huang, Y.; Fu, M.; Ho, W.K.; Zou, S.C.; Wang, B. Rose-Like Monodisperse Bismuth Subcarbonate Hierarchical Hollow Microspheres: One-Pot Template-Free Fabrication and Excellent Visible Light Photocatalytic Activity and Photochemical Stability for NO Removal in Indoor Air. J. Hazard. Mater. 2011, 195, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiong, T.; Zhao, Z.W.; Sun, Y.J.; Fu, M. Ammonia Induced Formation of N-Doped (BiO)2CO3 Hierarchical Microspheres: The Effect of Hydrothermal Temperature on the Morphology and Photocatalytic Activity. CrystEngComm 2013, 15, 10522–10532. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Cheng, G.; Lu, Z.; Tang, J.L.; Yu, X.L.; Chen, R. BiOCOOH hierarchical nanostructures: Shape-controlled solvothermal synthesis and photocatalytic degradation performances. CrystEngComm 2011, 13, 2381–2390. [Google Scholar] [CrossRef]
- Chai, B.; Wang, X. Enhanced visible light photocatalytic activity of BiOI/BiOCOOH composites synthesized via ion exchange strategy. RSC Adv. 2015, 5, 7589–7596. [Google Scholar] [CrossRef]
- Yang, L.L.; Han, Q.F.; Wang, X.; Zhu, J.W. Highly efficient removal of aqueous chromate and organic dyes by ultralong HCOOBiO nanowires. Chem. Eng. J. 2015, 262, 169–178. [Google Scholar] [CrossRef]
- Cheng, H.F.; Huang, B.B.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. A controlled anion exchange strategy to synthesize Bi2S3 nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity. Chem. Commun. 2012, 48, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Guan, M.L.; Liu, S.S.; Zhang, Y.Q.; Zhang, C.W.; He, Y.X.; Huang, S.M. Controlled synthesis of olive-shaped Bi2S3/BiVO4 microspheres through a limited chemical conversion route and enhanced visible-light-responding photocatalytic activity. Dalton Trans. 2012, 41, 5581–5586. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, B.Y.; Lin, H.L.; Luo, B.D.; Chen, S.F. Novel heterostructured Bi2S3/BiOI photocatalyst: Facile preparation, characterization and visible light photocatalytic performance. Dalton Trans. 2012, 41, 11482–11490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, S.F.; Luo, S.L.; Huang, R.; Zhang, Q.; Hong, T.; Au, P.C.T. Bi2O2CO3/BiOI Photocatalysts with Heterojunctions Highly Efficient for Visible-Light Treatment of Dye-Containing Wastewater. Ind. Eng. Chem. Res. 2012, 51, 6760–6768. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.J.; Fu, M.; Wu, Z.B.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219–220, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Zhang, Z.J.; Zhang, W.D. Inlay of Bi2O2CO3 nanoparticles onto Bi2WO6 nanosheets to build heterostructured photocatalysts. Dalton Trans. 2014, 43, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, J.X.; Song, Y.X.; Zhao, W.K.; Xu, Y.G.; Song, Y.H.; Ji, Y.H.; Li, H.M. Ion-exchange preparation for visible-light-driven photocatalyst AgBr/Ag2CO3 and its photocatalytic activity. RSC Adv. 2014, 4, 9139–9147. [Google Scholar] [CrossRef]
- Dong, H.J.; Chen, G.; Sun, J.X.; Feng, Y.J.; Li, C.M.; Xiong, G.H.; Lv, C.D. Highly-effective photocatalytic properties and interfacial transfer efficiencies of charge carriers for the novel Ag2CO3/AgX heterojunctions achieved by surface modification. Dalton Trans. 2014, 43, 7282–7289. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Z.Y.; Li, Y.H.; Ho, W.K.; Lee, S.C. Immobilization of Polymeric g-C3N4 on Structured Ceramic Foam for Efficient Visible Light Photocatalytic Air Purification with Real Indoor Illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.H.; Ho, W.K.; Lee, S.C.; Zhang, L.Z. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environ. Sci. Technol. 2009, 43, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiong, T.; Sun, Y.J.; Zhang, Y.X.; Zhou, Y. Controlling Interfacial Contact and Exposed Facets for Enhancing Photocatalysis via 2D-2D Heterostructure. Chem. Commun. 2015, 51, 8249–8252. [Google Scholar]
- Dong, F.; Xiong, T.; Sun, Y.J.; Huang, H.W.; Wu, Z.B. Synergistic Integration of Thermocatalysis and Photocatalysis on Black Defective (BiO)2CO3 Microspheres. J. Mater. Chem. A 2015, 3, 18466–18474. [Google Scholar] [CrossRef]
- Sample Availablity: Samples are available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Cui, W.; Zhong, J.; Liu, X.; Dong, F.; Zhang, Y. Enhanced Visible Light Photocatalytic Activity of Br-Doped Bismuth Oxide Formate Nanosheets. Molecules 2015, 20, 19189-19202. https://doi.org/10.3390/molecules201019189
Feng X, Cui W, Zhong J, Liu X, Dong F, Zhang Y. Enhanced Visible Light Photocatalytic Activity of Br-Doped Bismuth Oxide Formate Nanosheets. Molecules. 2015; 20(10):19189-19202. https://doi.org/10.3390/molecules201019189
Chicago/Turabian StyleFeng, Xin, Wen Cui, Junbo Zhong, Xiaoying Liu, Fan Dong, and Yuxin Zhang. 2015. "Enhanced Visible Light Photocatalytic Activity of Br-Doped Bismuth Oxide Formate Nanosheets" Molecules 20, no. 10: 19189-19202. https://doi.org/10.3390/molecules201019189





