1. Introduction
MicroRNAs (miRNAs) are 20–22-nucleotide-long, non-coding RNA molecules that post-transcriptionally regulate gene expression by base-pairing with the 3′ untranslated region of complementary messenger RNA targets [
1,
2]. The miRNA is associated with the RNA-induced silencing complex (RISC) [
3] and this complex binds target mRNAs through the complementary perfect region seed and partially complementary sequences tail and reduces their translation and/or stability [
4]. They regulate diverse biological processes, and bioinformatics data indicate that each miRNA can control hundreds of gene targets, underscoring the potential influence of miRNAs on almost every genetic pathway [
5,
6,
7,
8,
9,
10]. Growing evidence indicates that miRNAs exist not only in cells, but also in a variety of body fluids including blood [
11]. Extracellular miRNAs are easy to detect in body fluids, and they have been considered as potential biomarkers for specific diseases [
12]. The first studies revealed that these circulating miRNAs may be delivered to recipient cells where they can regulate translation of target genes [
13,
14]. While many studies have focused on the study of miRNA expression in physiological and pathological processes, various technical problems related to miRNA isolation have simultaneously emerged and the stability of the storage of miRNA in biological samples has been questioned [
13,
14]. The miRNAs in plasma can be quantitatively detected by methods such as real-time PCR and microarrays [
14]. Here we investigate the stability of miRNAs isolated from clinically healthy donors, and evaluate the abundance and solidity of miRNAs subjected to a long period of freezing. To assess the stability of the miRNAs, a real-time PCR analysis was performed on a panel of eight miRNAs from freshly isolated plasma samples and from samples subjected to six and 12 months of storage at −80 °C. Surprisingly, the expression of the tested miRNAs was stable for six and 12 months at −80 °C. miRNAs isolated from stored samples did not show any significant degradation. Moreover, the real-time PCR analysis was repeatedly performed on plasma samples stored over a period of ~14 years. The results showed that the samples were stable for four years and then began to decrease, remaining detectable. All samples were recruited from the biobank of the longevity AKeA Project (project approved by the local Ethics committee) [
15,
16].
2. Results and Discussion
The raw values, the mean and standard deviation of Ct (threshold cycle) of miRNAs and U6snRNA analyzed are reported in
supplementary Tables S1 and S2. Data for all targets resulted not normally distributed. The Kruskal-Wallis test showed no significant differences between years only for U6snRNA (
p = 0.977), miR200b-5p (
p = 0.099), miR-212-3p (
p = 0.082), and miR579-3p (
p = 0.079). The statistical analysis supported our decision to use the U6snRNA as normalizer for the real-time PCR analysis because of its greater stability between groups of samples. We analyzed eight miRNAs: miR-125b-5p; miR-425-5p; miR-200b-5p; miR-200c-3p; miR-579-3p; miR-212-3p; miR-126-3p; and miR-21-5p identified with a number from 1 to 8 in all graphics and tables. We performed a comparison between the level of miRNA in fresh plasma samples and the level of miRNA in frozen samples for different storage periods. In particular, the fresh samples were analyzed at the time of collection, and after six and 12 months of storage at −80 °C. miRNA levels in plasma samples collected in 1999, 2002, 2003, 2009, and 2010 and stored at −80 °C were also evaluated and compared to fresh samples. The Ct value of some samples frozen for six or 12 months showed to be less than that of paired fresh samples; this could be explained with technical error in the RNA extraction and/or RT-PCR procedure.
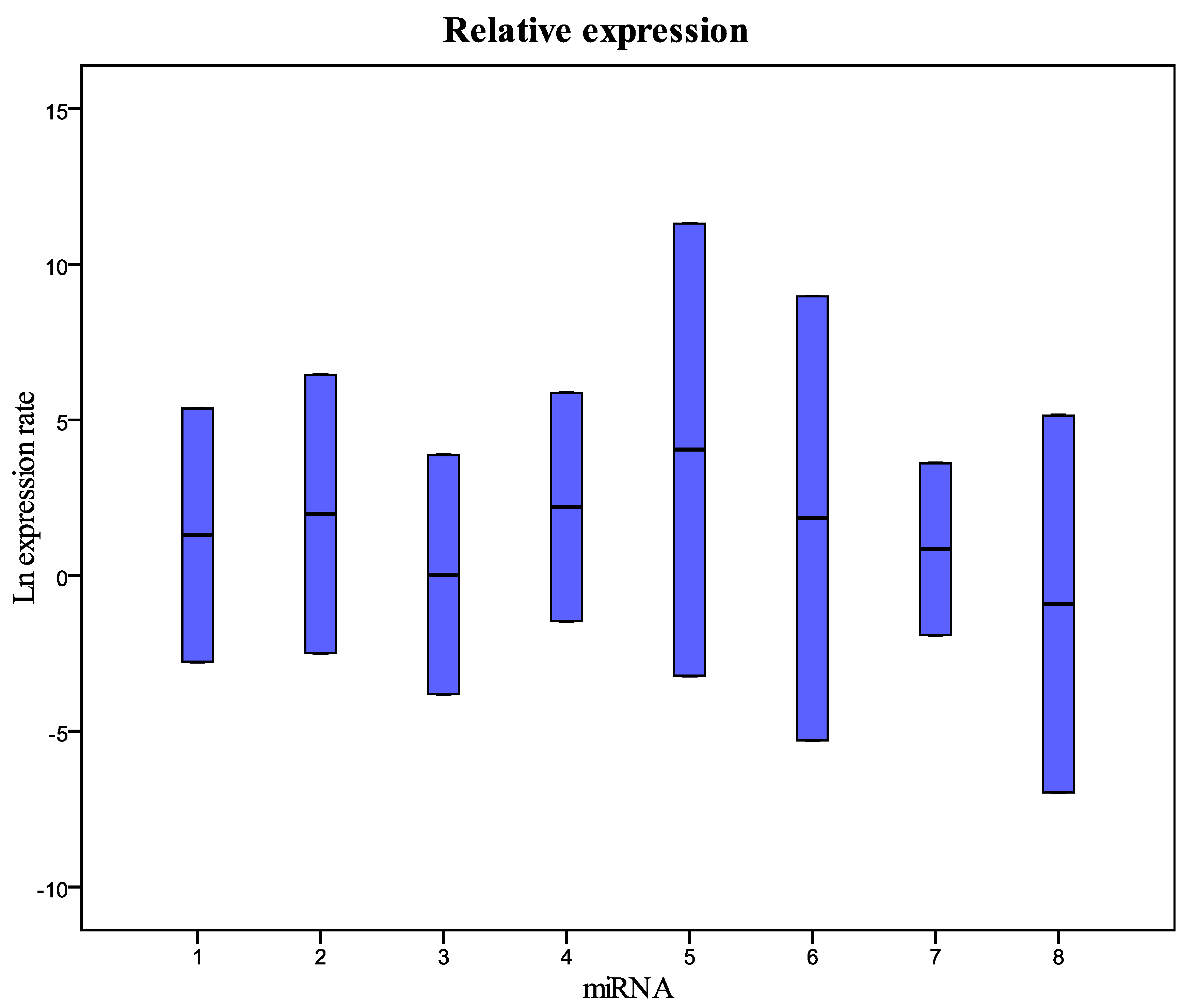
Figure 1 and
Table 1 show the relative concentration of the miRNAs analyzed in this study in the samples frozen for 12 months at −80 °C compared with fresh samples. As in the samples frozen for six months, these results show that there are no significant differences in the miRNA levels between fresh and frozen samples. The same results were obtained in the comparison between fresh samples and samples collected in 2010 and stored at −80 °C (
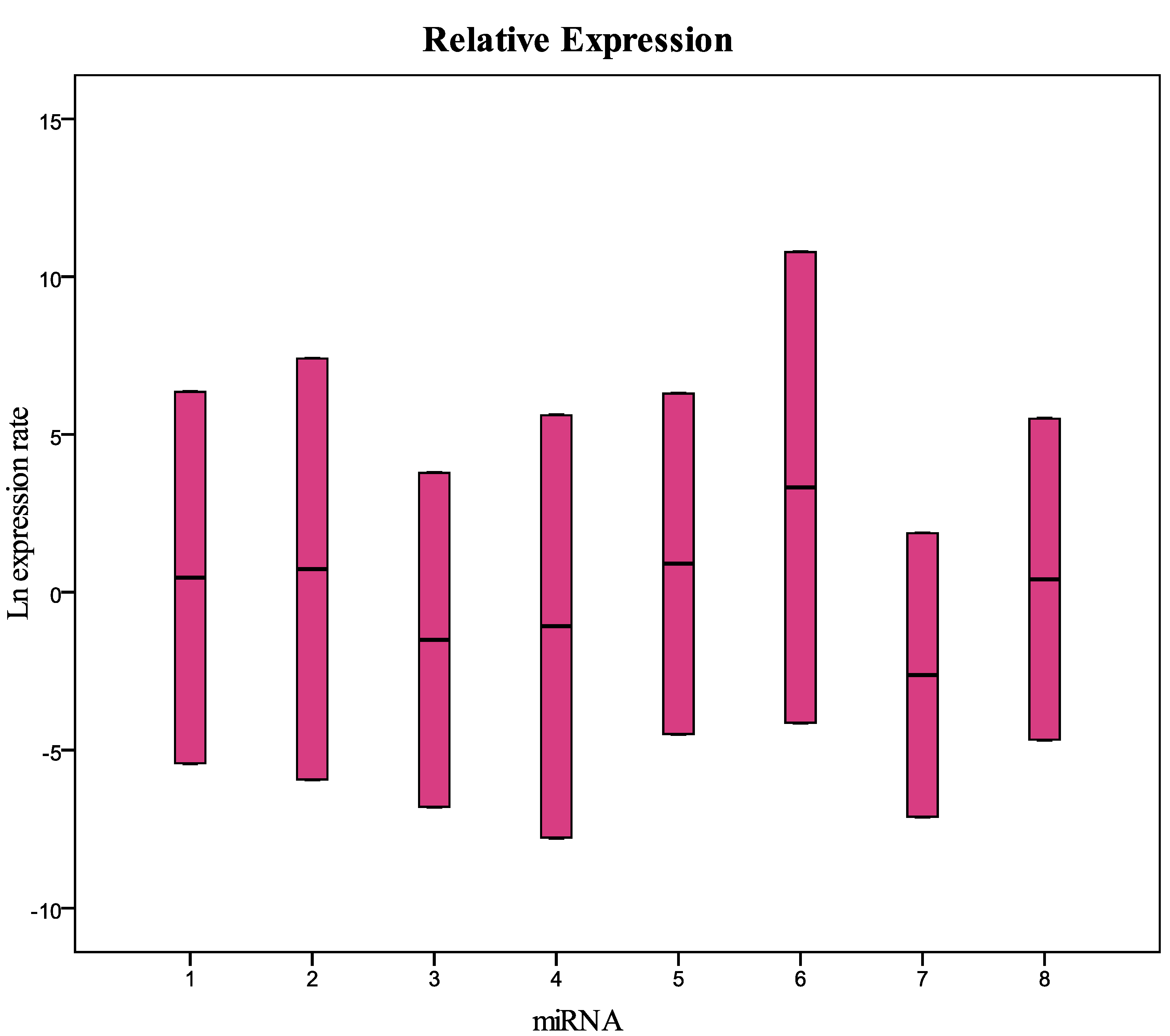
Figure 2 and
Table 2). The comparative analysis with the samples collected in 2002, 2003, and 2009 (
Figure 3 and
Table 3) showed that miRNA 126-3p (#7) had a concentration significantly lower (
p = 0.008) than fresh samples, while the other miRNAs showed no significant differences. Finally, in the samples collected in 1999, all miRNAs had a significantly low concentration, except for the miRNA 212-5p (#6) (
Figure 4 and
Table 4). These results show that the miRNAs decay at different times. During the first four years we did not observe significant differences in the concentration of miRNAs in fresh samples when compared to frozen samples. All miRNAs begin to decline after five years of freezing, while miR-212-3p does not decay and remains stable after 14 years of freezing. All miRNAs studied seem to show differences in stability in relation to the number of the AU or UA motifs in their sequences. We looked for a possible explanation for this result. In 2009, Sethi and Lukiw suggested a decline linked to the number of AU sequences in the miRNAs under investigation in their study [
17]. We observed this result in our samples subjected to freezing in the various time intervals. miRNA 212-3p has one AU sequence, all other miRNAs analyzed have from two to five AU sequences. miRNA 126-3p, which has five AU sequences, was the first to decrease its concentration after five years. We think that miRNA stability is related to the absence of AU sequences in seed and tail miRNA regions.
Table 1.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples frozen for 12 months compared with fresh samples). p-value (≥0.05) shows that there are no significant differences.
Table 1.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples frozen for 12 months compared with fresh samples). p-value (≥0.05) shows that there are no significant differences.
| miRNA | Type | Reaction Efficiency | Expression | Std. Error | 95% C.I. | P(H1) |
|---|
| 1 | TRG | 1 | 2.210 | 0.181–16.260 | 0.080–246.143 | 0.468 |
| 2 | TRG | 1 | 2.141 | 0.388–76.793 | 0.039–683.002 | 0.592 |
| 3 | TRG | 1 | 0.271 | 0.016–1.566 | 0.007–38.257 | 0.331 |
| 4 | TRG | 1 | 2.039 | 0.563–22.424 | 0.051–170.211 | 0.497 |
| 5 | TRG | 1 | 6.907 | 0.377–496.960 | 0.020–27.857 | 0.365 |
| 6 | TRG | 1 | 1.247 | 0.024–128.740 | 0.011–4.842 | 0.951 |
| 7 | TRG | 1 | 1.211 | 0.380–6.831 | 0.043–34.196 | 0.765 |
| 8 | TRG | 1 | 0.342 | 0.061–3.016 | 0.001–73.104 | 0.502 |
| U6snRNA | REF | 1 | 1 | | | |
Figure 1.
Relative expression (concentration) of the miRNAs analyzed in this study in samples frozen for 12 months compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Figure 1.
Relative expression (concentration) of the miRNAs analyzed in this study in samples frozen for 12 months compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Table 2.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 2010 and stored at −80 °C compared with fresh samples). p-value (≥0.05) shows that there are no significant differences.
Table 2.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 2010 and stored at −80 °C compared with fresh samples). p-value (≥0.05) shows that there are no significant differences.
| miRNA | Type | Reaction Efficiency | Expression | Std. Error | 95% C.I. | P(H1) |
|---|
| 1 | TRG | 1 | 6.088 | 0.376–118.479 | 0.169–5.649 | 0.300 |
| 2 | TRG | 1 | 3.430 | 0.237–96.389 | 0.132–11.035 | 0.543 |
| 3 | TRG | 1 | 0.205 | 0.001–58.136 | 0.001–1.239 | 0.496 |
| 4 | TRG | 1 | 1.273 | 0.060–76.914 | 0.031–4.285 | 0.915 |
| 5 | TRG | 1 | 2.900 | 0.205–55.911 | 0.125–3.116 | 0.526 |
| 6 | TRG | 1 | 7.825 | 0.441–1.673.136 | 0.115–14.474 | 0.315 |
| 7 | TRG | 1 | 0.373 | 0.089–2.646 | 0.050–11.195 | 0.254 |
| 8 | TRG | 1 | 3.317 | 0.210–148.920 | 0.144–5.319 | 0.536 |
| U6snRNA | REF | 1 | 1.000 | | | |
Figure 2.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 2010 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Figure 2.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 2010 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Table 3.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 2009 and stored at −80 °C compared with fresh samples). miRNA 126b-5p (#7) decreased significantly (p = 0.021) while all other miRNAs show no significant differences.
Table 3.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 2009 and stored at −80 °C compared with fresh samples). miRNA 126b-5p (#7) decreased significantly (p = 0.021) while all other miRNAs show no significant differences.
| miRNA | Type | Reaction Efficiency | Expression | Std. Error | 95% C.I. | P(H1) | Result |
|---|
| 1 | TRG | 1 | 1.446 | 0.075–22.084 | 0.005–399.228 | 0.762 | |
| 2 | TRG | 1 | 0.355 | 0.027–5.061 | 0.003–131.362 | 0.418 | |
| 3 | TRG | 1 | 0.110 | 0.002–2.424 | 0.002–79.778 | 0.120 | |
| 4 | TRG | 1 | 0.099 | 0.005–2.367 | 0.000–36.781 | 0.065 | |
| 5 | TRG | 1 | 0.308 | 0.014–7.950 | 0.000–156.536 | 0.468 | |
| 6 | TRG | 1 | 9.120 | 0.817–168.704 | 0.030–18.989 | 0.159 | |
| 7 | TRG | 1 | 0.093 | 0.020–0.479 | 0.001–5.080 | 0.008 | DOWN |
| 8 | TRG | 1 | 1.229 | 0.104–16.032 | 0.012–505.934 | 0.858 | |
| U6snRNA | REF | 1 | 1.000 | | | | |
Figure 3.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 2009 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Figure 3.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 2009 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Table 4.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 1999 and stored at −80 °C compared with fresh samples). All miRNAs decreased significantly, except miRNA-212-3p (#6) that shows no significant differences.
Table 4.
Relative expression (concentration), standard error, 95% confidence interval (C.I.), and p-value of miRNAs analyzed in this study (samples collected in 1999 and stored at −80 °C compared with fresh samples). All miRNAs decreased significantly, except miRNA-212-3p (#6) that shows no significant differences.
| miRNA | Type | Reaction Efficiency | Expression | Std. Error | 95% C.I. | P(H1) | Result |
|---|
| 1 | TRG | 1 | 0.009 | 0.000–0.326 | 0.000–15.647 | 0.007 | DOWN |
| 2 | TRG | 1 | 0.010 | 0.000–0.739 | 0.000–56.166 | 0.017 | DOWN |
| 3 | TRG | 1 | 0.024 | 0.003–0.372 | 0.000–10.244 | 0.008 | DOWN |
| 4 | TRG | 1 | 0.001 | 0.000–0.009 | 0.000–0.145 | 0.000 | DOWN |
| 5 | TRG | 1 | 0.008 | 0.000–0.838 | 0.000–39.763 | 0.021 | DOWN |
| 6 | TRG | 1 | 2.095 | 0.041–90.056 | 0.011–2.538 | 0.652 | |
| 7 | TRG | 1 | 0.059 | 0.005–0.882 | 0.002–10.576 | 0.007 | DOWN |
| 8 | TRG | 1 | 0.002 | 0.000–0.095 | 0.000–11.618 | 0.002 | DOWN |
| U6snRNA | REF | 1 | 1.000 | | | | |
Figure 4.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 1999 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.
Figure 4.
Relative expression (concentration) of the miRNAs analyzed in this study in samples collected in 1999 and frozen at −80 °C compared with fresh samples. 1 = miR-125b-5p; 2 = miR-425-5p; 3 = miR-200b-5p; 4 = miR-200c-3p; 5 = miR-579-3p; 6 = miR-212-3p; 7 = miR-126-3p; 8 = miR-21-5p.