Validated LC-MS/MS Method for the Determination of Scopoletin in Rat Plasma and Its Application to Pharmacokinetic Studies
Abstract
:1. Introduction
2. Results and Discussion
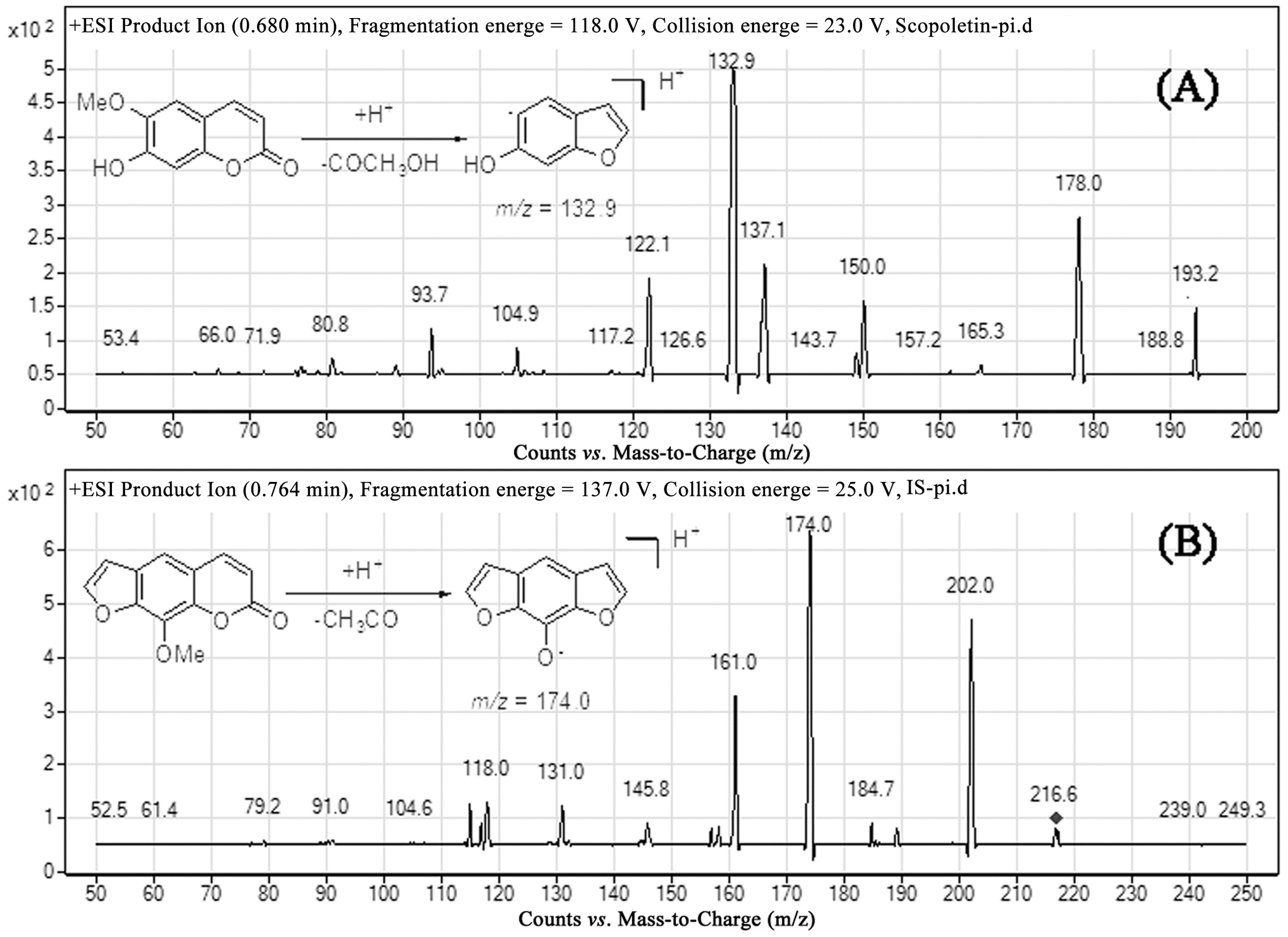
2.1. Optimization of Mass Spectrometric and Chromatographic Conditions
2.2. Optimization of Sample Preparation
2.3. Method Validation
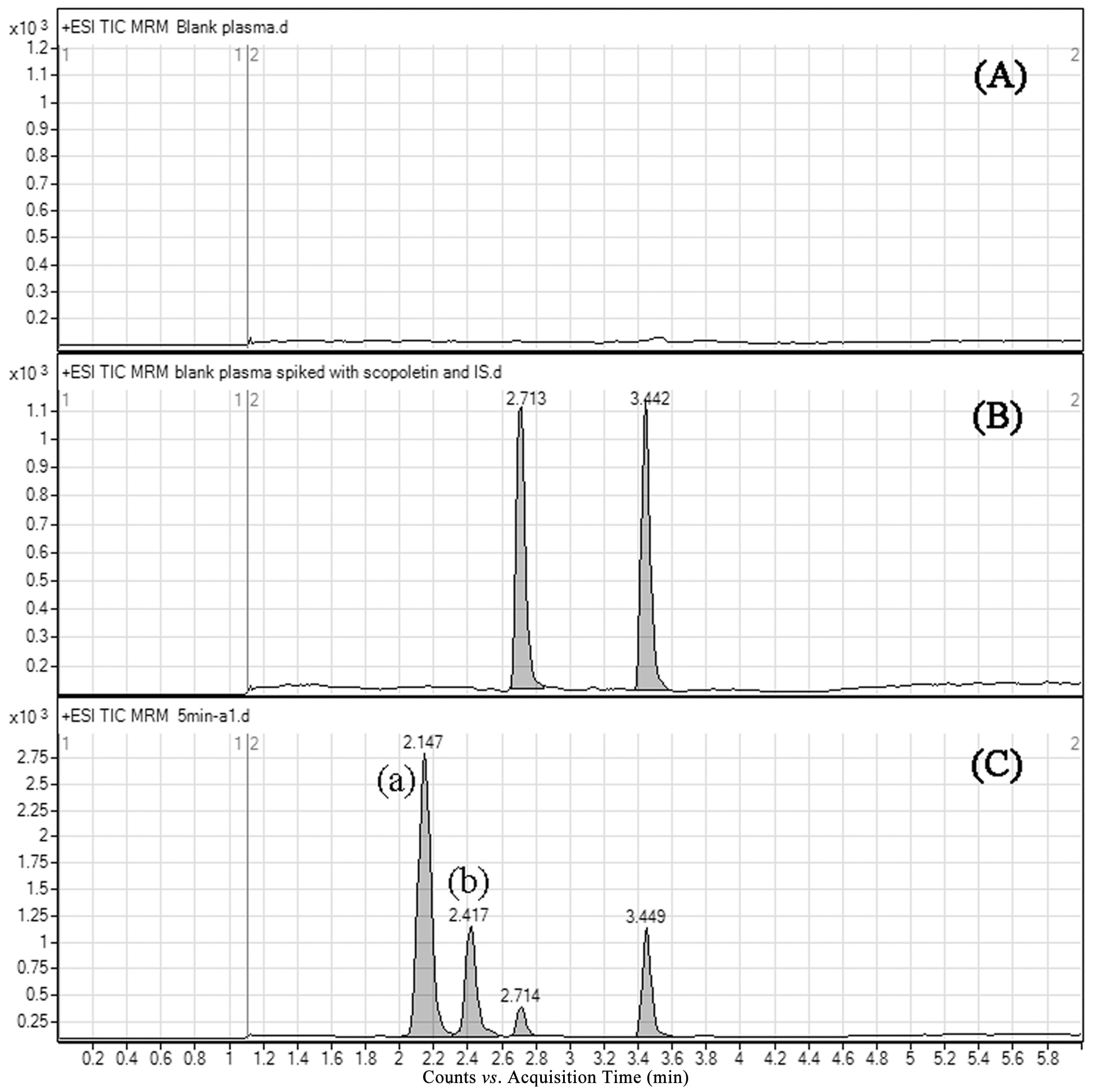
2.3.1. Selectivity
2.3.2. Linearity and Sensitivity
2.3.3. Extraction Recovery and Matrix Effect
| Compounds | Nominal Con. (ng/mL) | Recovery (Mean ± SD, %) | RSD (%) | Matrix Effect (Mean ± SD, %) | RSD (%) |
|---|---|---|---|---|---|
| Scopoletin | 10 | 96.6 ± 1.7 | 1.8 | 101.8 ± 5.3 | 5.2 |
| 100 | 93.9 ± 1.4 | 1.5 | 102.9 ± 3.2 | 3.1 | |
| 800 | 95.4 ± 0.7 | 0.7 | 103.0 ± 2.5 | 2.4 | |
| IS | 100 | 92.3 ± 1.3 | 1.4 | 97.4 ± 4.7 | 4.8 |
2.3.4. Precision and Accuracy
| Nominal Con. (ng/mL) | Intra-Day Run | Inter-Day Run | ||||
|---|---|---|---|---|---|---|
| Measured Con. (Mean ± SD, ng/mL) | RSD (%) | RE (%) | Measured Con. (Mean ± SD, ng/mL) | RSD (%) | RE (%) | |
| 10 | 9.70 ± 0.51 | 5.3 | −3.0 | 10.25 ± 0.62 | 6.1 | 2.5 |
| 100 | 100.48 ± 5.23 | 5.2 | 0.5 | 99.01 ± 3.68 | 3.7 | −0.1 |
| 800 | 818.62 ± 38.31 | 4.7 | 2.3 | 817.64 ± 26.56 | 3.2 | 2.2 |
2.3.5. Stability
| Storage Conditions | Nominal Con. (ng/mL) | Measured Con. (Mean ± SD, ng/mL) | RSD (%) | RE (%) |
|---|---|---|---|---|
| 3 freeze-thaw cycles | 10 | 10.36 ± 0.57 | 5.5 | 3.6 |
| 100 | 101.66 ± 3.99 | 3.9 | 1.7 | |
| 800 | 804.58 ± 6.13 | 0.8 | 0.6 | |
| Short-term stability for 6 h (25 °C) | 10 | 9.82 ± 0.49 | 5.0 | −1.8 |
| 100 | 101.39 ± 5.27 | 5.2 | 1.4 | |
| 800 | 812.33 ± 30.27 | 3.7 | 1.5 | |
| Long-term stability for 1 month (−20 °C) | 10 | 9.30 ± 0.45 | 4.8 | −7.0 |
| 100 | 98.20 ± 3.59 | 3.7 | −1.8 | |
| 800 | 813.78 ± 21.83 | 2.7 | 1.7 | |
| Long-term stability for 2 weeks (4 °C) | 10 | 9.80 ± 0.43 | 4.4 | −2.0 |
| 100 | 99.84 ± 2.97 | 3.0 | −0.2 | |
| 800 | 812.69 ± 21.48 | 2.6 | 1.6 | |
| Autosampler for 24 h (25 °C) | 10 | 9.83 ± 0.55 | 5.6 | −1.7 |
| 100 | 97.62 ± 4.40 | 4.5 | −2.4 | |
| 800 | 805.89 ± 24.31 | 3.0 | 0.7 |
2.3.6. Dilution Integrity
2.3.7. Incurred Sample Reanalysis
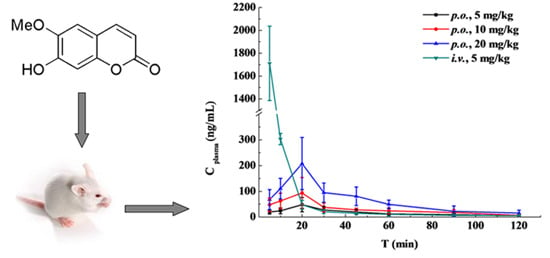
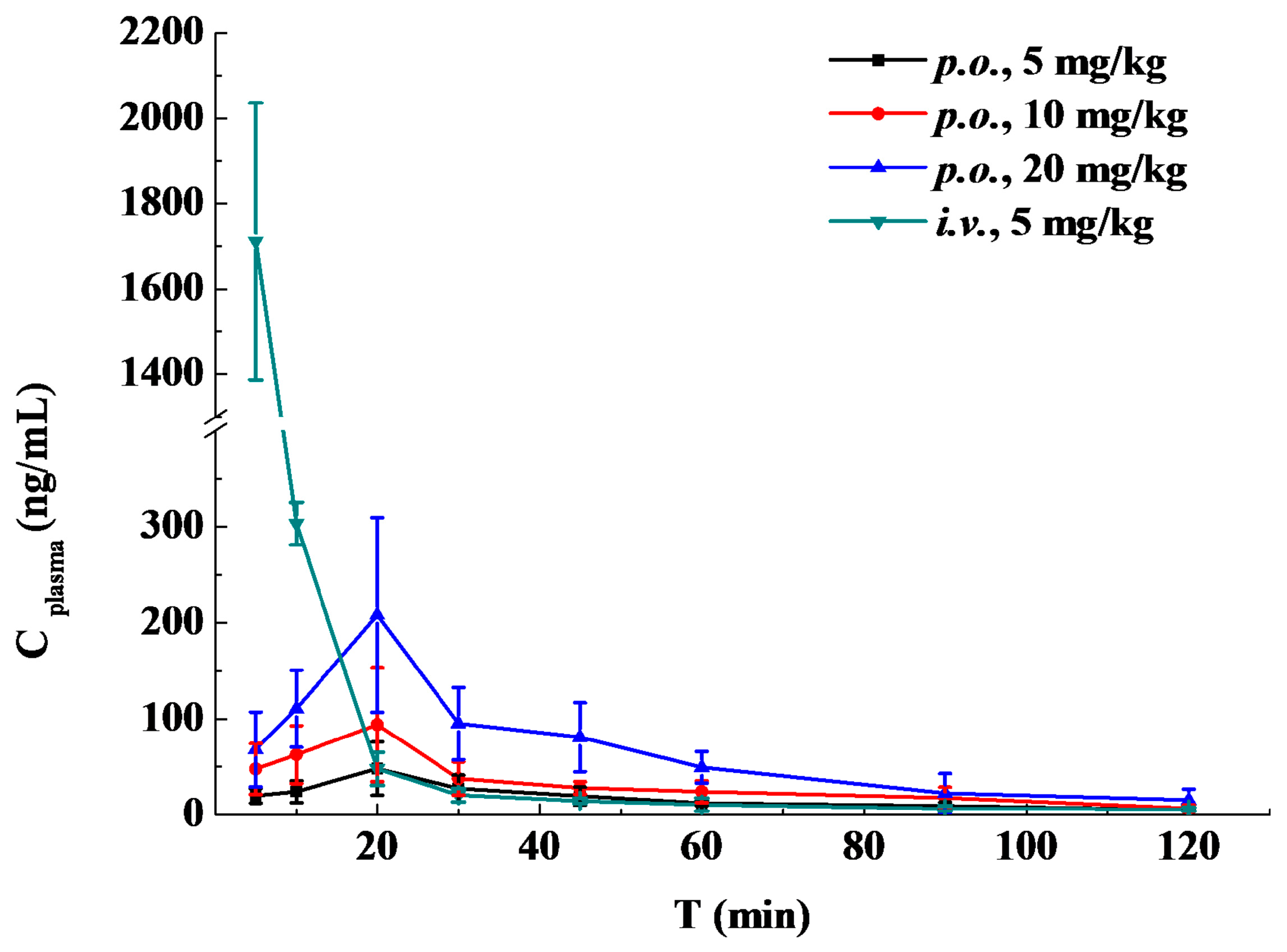
2.4. Pharmacokinetic Study and Oral Bioavailability
| t (h) | i.v. Administration | Oral Administration of the Indicated Dose | ||
|---|---|---|---|---|
| (5 mg/kg) | (5 mg/kg) | (10 mg/kg) | (20 mg/kg) | |
| 0.083 | 1711.13 ± 324.79 | 19.90 ± 7.70 | 47.80 ± 26.43 | 68.04 ± 38.88 |
| 0.167 | 303.00 ± 22.07 | 24.09 ± 11.36 | 62.61 ± 29.96 | 110.45 ± 40.16 |
| 0.33 | 47.98 ± 17.30 | 48.22 ± 27.74 | 93.85 ± 59.41 | 207.81 ± 101.26 |
| 0.5 | 20.58 ± 6.91 | 27.46 ± 14.06 | 37.95 ± 17.17 | 95.00 ± 37.45 |
| 0.75 | 14.45 ± 2.60 | 19.63 ± 9.48 | 28.09 ± 6.46 | 80.71 ± 35.88 |
| 1 | 10.67 ± 6.68 | 12.30 ± 2.94 | 24.22 ± 11.16 | 49.45 ± 16.46 |
| 1.5 | 6.31 ± 2.42 | 9.23 ± 3.98 | 17.68 ± 11.08 | 22.58 ± 20.41 |
| 2 | 5.79 ± 1.97 | 5.01 ± 1.38 | 6.86 ± 3.52 | 15.54 ± 11.34 |
| Parameter | i.v. Administration | Oral Administration of the Indicated Dose | ||
|---|---|---|---|---|
| (5 mg/kg) | (5 mg/kg) | (10 mg/kg) | (20 mg/kg) | |
| AUC0-t (μg /L·h) | 617.780 ± 188.945 | 33.819 ± 6.640 | 61.984 ± 9.406 | 127.770 ± 16.874 |
| AUC0-∞ (μg /L·h) | 624.855 ± 184.496 | 41.363 ± 12.048 | 69.910 ± 16.189 | 141.112 ± 20.857 |
| MRT0-t (h) | 0.066 ± 0.028 | 0.719 ± 0.102 | 0.685 ± 0.166 | 0.640 ± 0.164 |
| MRT0-∞ (h) | 0.116 ± 0.090 | 1.263 ± 0.700 | 0.948 ± 0.334 | 0.835 ± 0.299 |
| t1/2 (h) | 0.814 ± 0.326 | 0.935 ± 0.781 | 0.656 ± 0.379 | 0.539 ± 0.146 |
| Tmax (h) | NA | 0.398 ± 0.093 | 0.331 ± 0.118 | 0.414 ± 0.188 |
| CL (L/h/kg) | 8.661 ± 2.829 | 128.781 ± 34.852 | 148.364 ± 29.053 | 144.164 ± 20.623 |
| Cmax (μg/L) | NA | 49.786 ± 27.379 | 101.314 ± 52.201 | 217.324 ± 87.299 |
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Instrumentation and Analytical Conditions
3.3. Preparation of Stock and Working Solutions, Calibration Standards and Quality Control Samples
3.4. Sample Preparation
3.5. Method Validation
3.5.1. Selectivity
3.5.2. Linearity and Sensitivity
3.5.3. Extraction Recovery and Matrix Effect
3.5.4. Precision and Accuracy
3.5.5. Stability
3.5.6. Dilution Integrity
3.5.7. Incurred Sample Reanalysis
3.6. Application to Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The State Pharmacopoeia Commission of the P.R. China. Pharmacopoeia of the P.R. China; China Medical Science Press: Beijing, China, 2010; pp. 3–4. [Google Scholar]
- Shaw, C.Y.; Chen, C.H.; Hsu, C.C.; Chen, C.C.; Tsai, Y.C. Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother. Res. 2003, 17, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother. Res. 2006, 20, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.Q.; Dai, Y.; Wang, Z.T. Hypouricemic action of scopoletin arising from xanthine oxidase inhibition and uricosuric activity. Planta Med. 2005, 71, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Ardenghi, J.V.; Pretto, J.B.; Souza, M.M.; d’Avila Moura, J.; Junior, A.C.; Soldi, C.; Pizzolatti, M.G.; Santos, A.R. Antinociceptive properties of coumarins, steroid and dihydrostyryl-2-pyrones from Polygala sabulosa (Polygalaceae) in mice. J. Pharm. Pharmacol. 2006, 58, 107–112. [Google Scholar] [PubMed]
- Pan, R.; Gao, X.H.; Li, Y.; Xia, Y.F.; Dai, Y. Anti-arthritic effect of scopoletin, a coumarin compound occurring in Erycibe obtusifolia Benth stems, is associated with decreased angiogenesis in synovium. Fund. Clin. Pharmacol. 2010, 24, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jang, S.I.; Kim, Y.J.; Chung, H.T.; Yun, Y.G.; Kang, T.H.; Jeong, O.S.; Kim, Y.C. Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia 2004, 75, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IκB/NF-κB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.Q.; Dai, Y.; Hao, H.P.; Pan, R.; Yao, X.J.; Wang, Z.T. Anti-inflammatory effects of scopoletin and underlying mechanisms. Pharm. Biol. 2008, 46, 854–860. [Google Scholar] [CrossRef]
- Yao, X.J.; Ding, Z.Q.; Xia, Y.F.; Wei, Z.F.; Luo, Y.B.; Feleder, C.; Dai, Y. Inhibition of monosodium urate crystal-induced inflammation by scopoletin and underlying mechanisms. Int. Immunopharmacol. 2012, 14, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.F.; Dai, Y.; Wang, Q.; Liang, HZ. Determination of scopoletin in rat plasma by high performance liquid chromatographic method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. B 2007, 857, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, X.W.; Yang, W.; Liu, S.C.; Wang, N.; Shi, R.; Qiao, S.; Wang, Q.; Wang, Y.L. Quantitative analysis of nine coumarins in rat urine and bile after oral administration of Radix Glehniae extract by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Zhang, Q.H.; Li, J.; Zhang, L.; Guo, X.R.; He, J.; Zhang, P.; Ma, L.; Deng, Y.R.; Zhang, B.L.; Gao, X.M. Simultaneous determination of scopoletin, psoralen, bergapten, xanthotoxin, columbianetin acetate, imperatorin, osthole and isoimperatorin in rat plasma by LC-MS/MS for pharmacokinetic studies following oral administration of Radix Angelicae Pubescentis extract. J. Pharm. Biomed. Anal. 2013, 77, 71–75. [Google Scholar] [PubMed]
- Yi, T.; Zhu, L.; Tang, Y.N.; Zhang, J.Y.; Liang, Z.T.; Xu, J.; Zhao, Z.Z.; Yu, Z.L.; Bian, Z.X.; Yang, Z.J.; et al. An integrated strategy based on UPLC-DAD-QTOF-MS for metabolism and pharmacokinetic studies of herbal medicines: Tibetan “Snow Lotus” herb (Saussurea laniceps), a case study. J. Ethnopharmacol. 2014, 153, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cai, D.; Bi, H.; Zhong, G.; Zeng, H.; Gu, L.; Huang, Z.; Huang, M. Comparative pharmacokinetics of paclitaxel after oral administration of Taxus yunnanensis extract and pure paclitaxel to rats. Fitoterapia 2013, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- EMA and CHMP, Guideline on Bioanalytical Method Validation. European Medicines Agency and Committee for Medicinal Products for Human Use. 2011. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (accessed on 15 April 2014).
- US DHHS, FDA and CDER. Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2013. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf (accessed on 15 April 2014). [Google Scholar]
- Ding, W.J.; Deng, Y.; Feng, H.; Liu, W.W.; Hu, R.; Li, X.; Gu, Z.M.; Dong, X.P. Biotransformation of aesculin by human gut bacteria and identification of its metabolites in rat urine. World J. Gastroenterol. 2015, 12, 1518–1523. [Google Scholar] [CrossRef]
- Ritschel, W.A.; Brady, M.E.; Tan, H.I.S.; Hoffmann, K.A.; Yiu, I.M.; Grummich, K.W. Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur. J. Clin. Pharmacol. 1977, 12, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, W.A.; Brady, M.E.; Tan, H.I.S. First-pass effect of coumarin in man. Int. J. Clin. Pharmacol. Biopharm. 1978, 17, 99–103. [Google Scholar]
- Lin, Q.; Zhang, Q.; Song, X.; Gong, T.; Sun, X.; Zhang, Z.R. Novel LC-MS/MS method for analyzing imperialine in rat plasma: Development, validation, and application to pharmacokinetics. J. Chromatogr. B 2013, 938, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds scopoletin are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Li, S.; Wang, X.; Gong, T.; Sun, X.; Zhang, Z. Validated LC-MS/MS Method for the Determination of Scopoletin in Rat Plasma and Its Application to Pharmacokinetic Studies. Molecules 2015, 20, 18988-19001. https://doi.org/10.3390/molecules201018988
Zeng Y, Li S, Wang X, Gong T, Sun X, Zhang Z. Validated LC-MS/MS Method for the Determination of Scopoletin in Rat Plasma and Its Application to Pharmacokinetic Studies. Molecules. 2015; 20(10):18988-19001. https://doi.org/10.3390/molecules201018988
Chicago/Turabian StyleZeng, Yingchun, Sha Li, Xiaohong Wang, Tao Gong, Xun Sun, and Zhirong Zhang. 2015. "Validated LC-MS/MS Method for the Determination of Scopoletin in Rat Plasma and Its Application to Pharmacokinetic Studies" Molecules 20, no. 10: 18988-19001. https://doi.org/10.3390/molecules201018988