Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad
Abstract
:1. Introduction
2. Results and Discussion
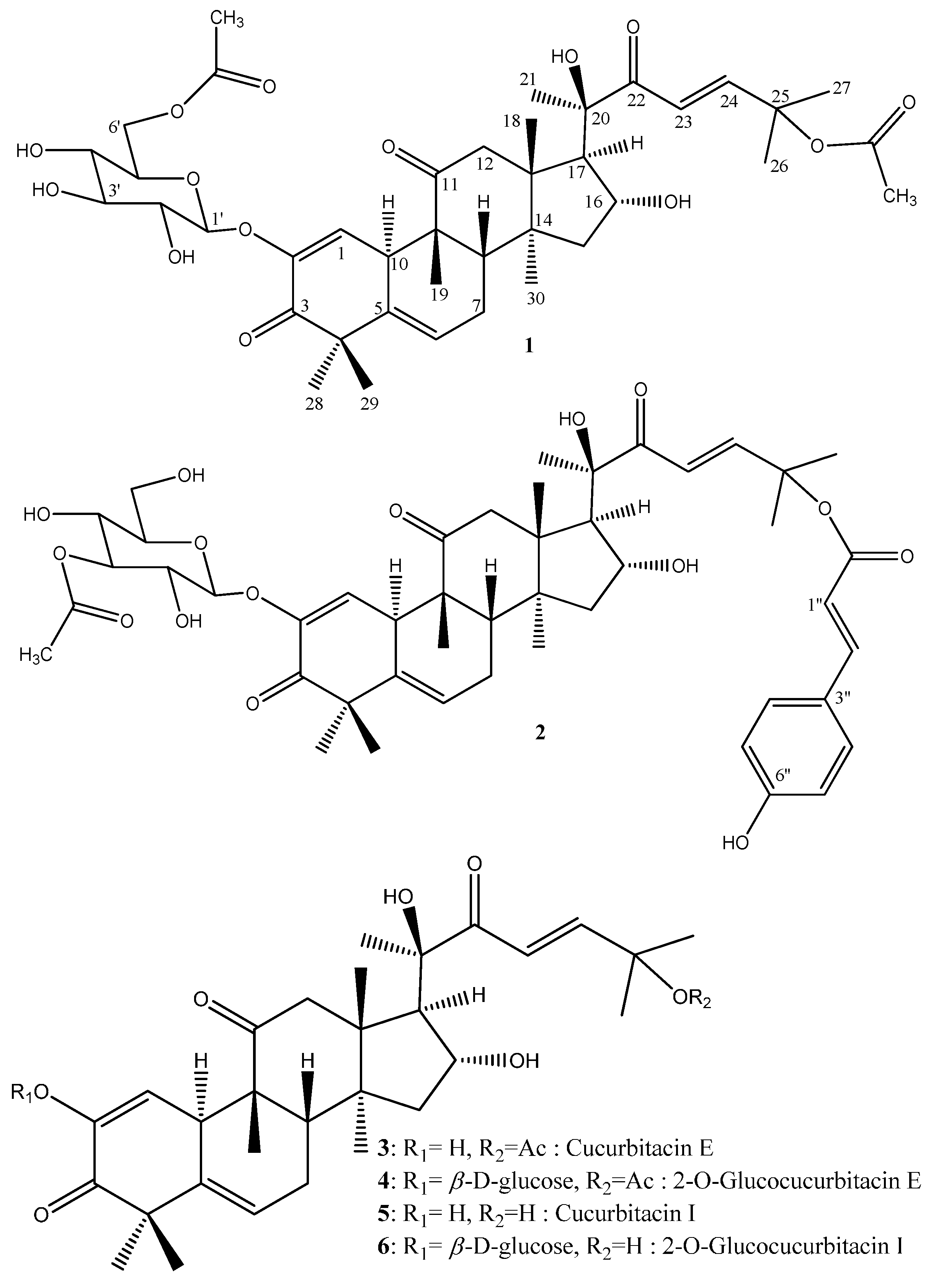
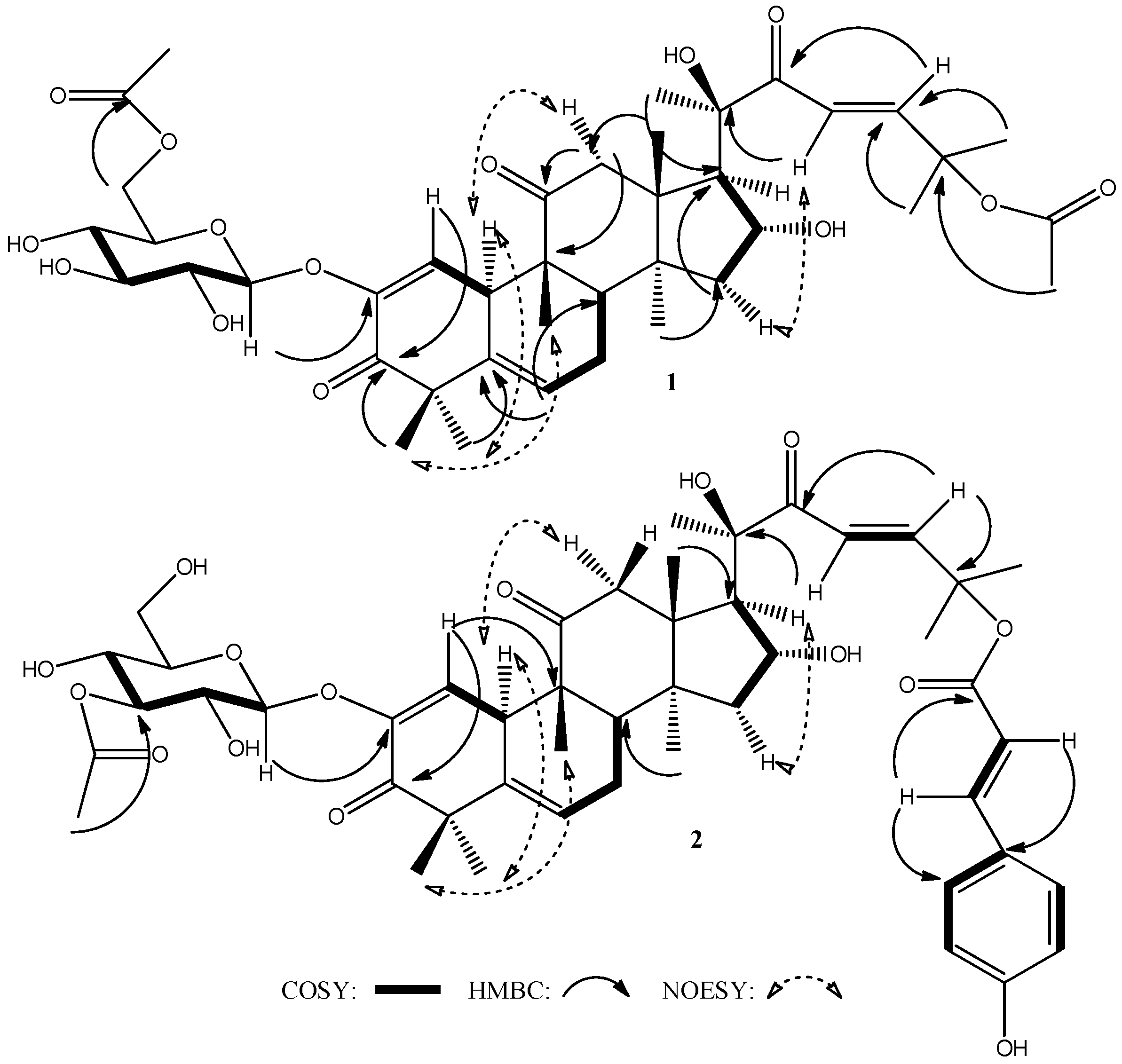
| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | Type | δH (J in Hz) | δC | Type | |
| 1 | 6.21 d (2.0) | 123.8 | CH | 6.11 d (2.3) | 121.5 | CH |
| 2 | 145.6 | C | 145.9 | C | ||
| 3 | 198.2 | C | 198.2 | C | ||
| 4 | 48.6 | C | 48.9 | C | ||
| 5 | 135.8 | C | 136.8 | C | ||
| 6 | 5.80 m | 121.2 | CH | 5.83 m | 120.4 | CH |
| 7a | 2.41 dd (11.6, 7.2) | 23.6 | CH2 | 2.09 m | 23.2 | CH2 |
| 7b | 2.06 d (11.6) | 2.36 m | ||||
| 8 | 2.05 m | 41.5 | CH | 2.02 m | 41.9 | CH |
| 9 | 48.7 | C | 48.7 | C | ||
| 10 | 3.50 brs | 35.3 | CH | 3.67 brs | 35.1 | CH |
| 11 | 213.1 | C | 214.8 | C | ||
| 12a | 2.74 d (14.7) | 48.9 | CH2 | 2.62 d (14.8) | 47.9 | CH2 |
| 12b | 3.26 d (14.7) | 3.32 d (14.8) | ||||
| 13 | 50.4 | C | 48.5 | C | ||
| 14 | 48.1 | C | 50.7 | C | ||
| 15a | 1.49 d (12.9) | 45.5 | CH2 | 1.45 m | 45.1 | CH2 |
| 15b | 1.91 dd (12.9, 3.2) | 1.87 m | ||||
| 16 | 4.40 ddd (3.2, 3.6, 7.2) | 71.2 | CH | 4.59 m | 70.5 | CH |
| 17 | 2.50 d (7.2) | 58.2 | CH | 2.59 d (7.0) | 58.7 | CH |
| 18 | 1.01 s | 19.9 | CH3 | 0.89 s | 19.5 | CH3 |
| 19 | 1.05 s | 20.2 | CH3 | 0.99 s | 19.3 | CH3 |
| 20 | 78.2 | C | 78.9 | C | ||
| 21 | 1.45 s | 23.9 | CH | 1.43 s | 24.0 | CH3 |
| 22 | 202.5 | C | 203.9 | C | ||
| 23 | 6.49 d (15.7) | 120.5 | CH | 6.86 d (16.0) | 121.3 | CH |
| 24 | 7.07 d (15.7) | 152.0 | CH | 7.01 d (16.0) | 150.5 | CH |
| 25 | 79.3 | C | 79.7 | C | ||
| 26 | 1.56 s | 26.4 | CH3 | 1.56 s | 25.5 | CH3 |
| 27 | 1.59 s | 25.8 | CH3 | 1.58 s | 25.1 | CH3 |
| 28 | 1.32 s | 20.3 | CH3 | 1.31 s | 19.5 | CH3 |
| 29 | 1.25 s | 27.8 | CH3 | 1.27 s | 26.8 | CH3 |
| 30 | 1.40 s | 18.3 | CH3 | 1.40 s | 17.4 | CH3 |
| CH3-CO | 2.03 s | 22.0 | CH3 | 2.01 s | 20.6 | CH3 |
| CH3-CO | 170.3 | C | 170.6 | C | ||
| CH3-CO | 2.12 s | 21.0 | CH3 | |||
| CH3-CO | 171.9 | C | ||||
| 1′ | 4.65 d (7.7) | 100.0 | CH | 4.70 d (7.2) | 99.7 | CH |
| 2′ | 3.55 m | 72.6 | CH | 3.44 dd (7.2) | 72,9 | CH |
| 3′ | 3.61 m | 74.5 | CH | 3.46 m | 74.6 | CH |
| 4′ | 3.66 m | 75.2 | CH | 3.41 m | 69.6 | CH |
| 5′ | 3.50 m | 69.2 | CH | 3.70 m | 75.9 | CH |
| 6′a | 4.20 dd (12.9, 3.8) | 65.4 | CH2 | 4.36 m | 62.9 | CH2 |
| 6′b | 4.55 dd (12.8, 4.0) | 4.68 m | ||||
| C=O | 169.3 | C | ||||
| 1″ | 6.50 d (15.8) | 114.0 | CH | |||
| 2″ | 7.64 d (15.8) | 145.8 | CH | |||
| 3″ | 126.4 | C | ||||
| 4″, 8″ | 7.43 d (8.7) | 129.8 | CH | |||
| 5″, 7″ | 6.80 d (8.7) | 115.7 | CH | |||
| 6″ | 161.2 | C | ||||

| µg/mL | HT29 (% of Variation vs. Control, Mean ± SD) | Caco-2 (% of Variation vs. Control, Mean ± SD) | IEC6 (% of Variation vs. Control, Mean ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 50 | 100 | 1 | 10 | 50 | 100 | 1 | 10 | 50 | 100 | |
| 2D culture (3 assays) | ||||||||||||
| Acetyl Glucocucurbitacin E (1) | 4.9 ± 0.1 | −7.4 ± 0 | −4.1 ± 0.1 | −0.2 ± 0 | −6.7 ± 0.1 | −45.5 ± 0.1 | −55.4 ± 0 | −53.7 ± 0 | −6.7 ± 0 | 0 ± 0 | 15.5 ± 0 | 23.2 ± 0 |
| Coumaroyl-acetyl Glucocucurbitacin I (2) | −6.4 ± 0.1 | −4.1 ± 0 | −4.7 ± 0.1 | −3.8 ± 0.1 | −47.7 ± 0.1 | −45.2 ± 0.1 | −52.1 ± 0 | −35.8 ± 0 | −23.7 ± 0 | −24.7 ± 0 | −2.2 ± 0 | 7.3 ± 0 |
| Cucurbitacin E (3) | 8.5 ± 0.2 | 4.8 ± 0 | 13.9 ± 0 | 16.4 ± 0 | −11.4 ± 0 | 7.1 ± 0.1 | −8.0 ± 0 | −0.3 ± 0.1 | −1.2 ± 0 | 7.7 ± 0 | 20.9 ± 0 | 27.9 ± 0 |
| Glucocucurbitacin E (4) | 11.4 ± 0.1 | −0.1 ± 0.1 | −9.7 ± 0.2 | 7.5 ± 0.1 | −2.7 ± 0.2 | 11.1 ± 0.1 | 4.4 ± 0 | −1.1 ± 0 | 8.1 ± 0 | 7.2 ± 0 | 36.0 ± 0 | 29.3 ± 0 |
| Cucurbitacin I (5) | 18.8 ± 0.1 | 6.3 ± 0 | 5.8 ± 0.1 | 10.7 ± 0 | −11.0 ± 0.1 | 1.9 ± 0.1 | −3.1 ± 0 | −1.4 ± 0 | −1.8 ± 0 | 8.7 ± 0.1 | 2.2 ± 0.1 | −14.0 ± 0.1 |
| Glucocucurbitacin I (6) | 2.7 ± 0.1 | 2.3 ± 0 | −1.6 ± 0.1 | 2.8 ± 0 | −2.6 ± 0.1 | −45.3 ± 0.1 | −49.1 ± 0 | −55.4 ± 0 | −0.8 ± 0 | 4.0 ± 0 | 10.7 ± 0 | 16.3 ± 0.1 |
| 3D culture (2 assays) | ||||||||||||
| Acetyl Glucocucurbitacin E (1) | 0 ± 0.1 | −24.0 ± 0.2 | −6.2 ± 0.4 | nd | −3.5 ± 0.4 | −3.3 ± 0.1 | −23.0 ± 0.1 | nd | −13.2 ± 0.1 | 2.0 ± 0 | −17.8 ± 0.3 | nd |
| Coumaroyl-acetyl Glucocucurbitacin I (2) | −32.5 ± 0.1 | −31.9 ± 0.1 | −51.7 ± 0.1 | nd | −19.0 ± 0.2 | −10.1 ± 0.1 | −54.0 ± 0.1 | nd | 5.7 ± 0.3 | 2.0 ± 0 | −24.4 ± 0.1 | nd |
| Glucocucurbitacin I (6) | 23.2 ± 0.1 | 1.2 ± 0.3 | 1.8 ± 0.4 | nd | −1.5 ± 0.2 | −1.7 ± 0.4 | −12.1 ± 0.2 | nd | −2.6 ± 0.1 | −5.4 ± 0.1 | −9.1 ± 0.1 | nd |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Physical Data of New Compounds
3.5. Cytotoxicity Assay
3.6. LC-MS Profiles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kammoun, M.; Koubaa, I.; Ben Ali, Y.; Jarraya, R.; Gargouri, Y.; Damak, M.; Bezzine, S. Inhibition of pro-inflammatory secreted phospholipase A2 by extracts from Cynara cardunculus L. Appl. Biochem. Biotechnol. 2010, 162, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Jarraya, R.; Lassoued, I.; Masmoudi, O.; Damak, M.; Nasri, M. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochem. 2010, 45, 1486–1493. [Google Scholar] [CrossRef]
- Hammami, H.; Jarraya, R.M.; Damak, M.; Ayadi, A. Molluscicidal activity of various solvent extracts of Solanum nigrum var. villosum L. aerial parts against Galba truncatula. Parasite 2011, 18, 63–70. [Google Scholar] [PubMed]
- Bourogaa, E.; Bertrand, J.; Despeaux, M.; Jarraya, R.; Fabre, N.; Payrastre, L.; Demur, C.; Fournier, J.J.; Damak, M.; el Feki, A.; et al. Hammada scoparia Flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk. Res. 2011, 35, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Trigui, M.; Ben Mansour, R.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Marzouk, B.; Marzouk, Z.; Décor, R.; Edziri, H.; Haloui, E.; Fenina, N.; Aouni, M. Antibacterial and anticandidal screening of Tunisian Citrullus colocynthis Schrad. from Medenine. J. Ethnopharmacol. 2009, 125, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, B.; Marzouk, Z.; Décor, R.; Edziri, H.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Lee, S.E.; Yang, J.Y.; Lee, H.S. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues against foodborne bacteria. J. Sci. Food Agric. 2014, 94, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Huseini, F.H.; Darvishzadeh, G.; Heshmat, R.; Jafariazar, Z.; Raza, M.; Larijai, B. The clinical investigation of Citrullus colocynthis (L.) Schrad fruit in treatment of type 2 diabetic patients: A randomized, double bind, placebo-controlled clinical trial. Phytother. Res. 2009, 23, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Sanadgol, N.; Nejad, B.S.; Beiragi, M.A.; Snadgol, E. Phytochemical screening and antibacterial activity of Citrullus colocynthis (Linn.) Schrad against Staphylococcus aureus. J. Med. Plant Res. 2010, 4, 2321–2325. [Google Scholar]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.A.; Chatha, S.A.S.; Sarker, S.D.; Gilani, A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.S.; Priyavardhini, S.; Vasantha, K.; Umadevi, M. Antibacterial activity on Citrullus colocynthis leaf extract. Anc. Sci. Life 2009, 29, 12–13. [Google Scholar] [PubMed]
- Nessa, F.; Khan, S.A. Evaluation of antioxidant and xanthine oxidase inhibitory activity of different solvent extracts of leaves of Citrullus colocynthis. Pharmacogn. Res. 2014, 6, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Uma, C.; Sekar, K.G. Phytochemical analysis of a folklore medicinal plant Citrullus colocynthis L. (bitter apple). J. Pharmacogn. Phytochem. 2014, 2, 195–202. [Google Scholar]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.A.; Chatha, S.A.S.; Ahmad, F.; Ahmad, A.; Johns, E.J. Phenolic profile and antioxidant activity of various extracts from Citrullus colocynthis (L.) from the Pakistani flora. Ind. Crop. Prod. 2013, 45, 416–422. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, H.S. Biofunctional constituent isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues show acaricidal and insecticidal efficacy. J. Agric. Food Chem. 2014, 62, 8663–8667. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Koike, K.; Tatsuzaki, M.; Koide, A.; Nikaido, T. Cucurbitosides F-M, acylated phenolic glycosides from the seeds of Cucurbita pepo. J. Nat. Prod. 2005, 68, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Dai, B.; Zhang, H.Y.; Xie, J.W.; Gu, C.Z.; Zhang, J. Two new cucurbitane-type triterpenoid saponins isolated from ethyl acetate extract of Citrullus colocynthis fruit. J. Asian Nat. Prod. Res. 2015, 11. [Google Scholar] [CrossRef]
- Nayab, D.; Ali, D.; Arshad, N.; Malik, A.; Choudhary, M.I.; Ahmed, Z. Cucurbitacin glucosides from Citrullus colocynthis. Nat. Prod. Res. 2006, 20, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Al-Yahya, M.; Al-Farhan, A. Response of Najdi sheep to oral administration of Citrullus colocynthis fruits. Small Rumin. Res. 2001, 40, 239–244. [Google Scholar] [CrossRef]
- Sturm, S.; Schveider, P.; Seger, C.; Stuppner, H. Analysis of Citrullus colocynthis cucurbitacin derivatives with HPLC-SPE-NMR. Sci. Pharm. 2009, 77, 254–257. [Google Scholar] [CrossRef]
- Hatam, N.A.; Whiting, D.A.; Yousif, N.J. Cucurbitacin glycosides from Citrullus colocynthis. Phytochemistry 1989, 28, 1268–1271. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S.; Mair, M.; Ellmerer, E.; Stuppner, H. 1H- and 13C-NMR signal assignment of cucurbitacin derivatives from Citrullus colocynthis (L.) Schrader and Ecballium elaterium (L.) (Cucurbitaceae). Magn. Reson. Chem. 2005, 43, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Iwanski, G.B.; Thoennissen, N.H. Cucurbitacin: Ancient compound shedding new light on cancer treatment. Sci. World J. 2010, 10, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Andújar, I.; Escandell, J.M.; Giner, R.M.; Recio, M.C. Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds. Curr. Pharm. Des. 2012, 18, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bao, J.; Guo, J.; Ding, Q.; Lu, J.; Huang, M.; Wang, Y. Biological activities and potential molecular targets of cucurbitacins: A focus on cancer. Anticancer Drugs 2012, 23, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Alghasham, A.A. Cucurbitacins—A promising target for cancer therapy. Int. J. Health Sci. 2013, 7, 77–89. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef]
- Abbas, S.; Vincourt, J.B.; Habib, L.; Netter, P.; Greige-Gerges, H.; Magdalou, J. The cucurbitacins E, D and I: Investigation of their cytotoxicity toward human chondrosarcoma SW 1353 cell line and their biotransformation in man liver. Toxicol. Lett. 2013, 216, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.H.; Kim, J.K. Cucurbitacin-I, a natural cell-permeable triterpenoid isolated from Cucurbitaceae, exerts potent anticancer effect in colon cancer. Chem. Biol. Interact. 2014, 219, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; Mersch, S.; Deenen, R.; Schmidt, S.; Messner, I.; Schafer, K.L.; Baldus, S.E.; Huckenbeck, W.; Piekorz, R.P.; Knoefel, W.T.; et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS ONE 2013, 8, e59689. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Shimura, H.; Sasahira, T.; Fujii, K.; Kuniyasu, H. High concentration of deoxycholic acid abrogates in vitro transformation of IEC6 intestinal cells by azoxymethane. J. Exp. Clin. Cancer Res. 2005, 24, 625–631. [Google Scholar] [PubMed]
- Vande Velde, V.; Lavie, D. 13C-NMR spectroscopy of cucurbitacins. Tetrahedron 1983, 39, 317–321. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Kobayashi, H.; Nakamura, A.; Matsuhira, K.; Nakamura, S.; Matsuda, H. Bioactive saponins and glycosides. XXVII. Structures of new cucurbitane-type triterpene glycosides and antiallergic constituents from Citrullus colocynthis. Chem. Pharm. Bull. 2007, 55, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ponosyan, A.G.; Nikishchenko, M.N.; Avetisyan, G.M. Structure of 22-deoxocucurbitacins isolated from Bryonia alba and Ecballium elaterium. Chem. Nat. Compd. 1985, 21, 638–645. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products. A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Chichester, UK, 2009; p. 239. [Google Scholar]
- Zahir, N.; Weaver, V.M. Death in the third dimension: Apoptosis regulation and tissue architecture. Curr. Opin. Genet. Dev. 2004, 14, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, H.; Li, Z.; Yang, C.; Wang, C. Cucurbitacin I inhibits cell migration and invasion and enhances chemosensitivity in colon cancer. Oncol. Rep. 2015, 33, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Escandell, J.M.; Kaler, P.; Recio, M.C.; Sasazuki, T.; Shirasawa, S.; Augenlicht, L.; Rios, J.L.; Klampfer, L. Activated kRas protects colon cancer cells from cucurbitacin-induced apoptosis: The role of p53 and p21. Biochem. Pharmacol. 2008, 76, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawech, R.; Jarraya, R.; Girardi, C.; Vansteelandt, M.; Marti, G.; Nasri, I.; Racaud-Sultan, C.; Fabre, N. Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules 2015, 20, 18001-18015. https://doi.org/10.3390/molecules201018001
Chawech R, Jarraya R, Girardi C, Vansteelandt M, Marti G, Nasri I, Racaud-Sultan C, Fabre N. Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules. 2015; 20(10):18001-18015. https://doi.org/10.3390/molecules201018001
Chicago/Turabian StyleChawech, Rachid, Raoudha Jarraya, Cynthia Girardi, Marieke Vansteelandt, Guillaume Marti, Imen Nasri, Claire Racaud-Sultan, and Nicolas Fabre. 2015. "Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad" Molecules 20, no. 10: 18001-18015. https://doi.org/10.3390/molecules201018001





