In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity
| EC50 Value a (mg/mL) | ||||
|---|---|---|---|---|
| Scavenging Ability on DPPH Radicals | Scavenging Ability on OH Radicals | Chelating Ability on Ferrous Ions | Reducing Power | |
| MCE | 0.29 ± 0.14 b | 6.10 ± 0.01 a | 4.34 ± 0.08 a | 2.90 ± 0.01 c |
| ECE | 0.97 ± 0.22 b | 2.90 ± 0.08 c | 1.49 ± 0.07 c | 4.92 ± 0.03 b |
| ACE | 2.95 ± 0.71 a | 4.26 ± 0.06 b | 1.77 ± 0.08 b | 16.42 ± 0.04 a |
| Polysaccharide | 0.02 ± 0.01 b | 2.79 ± 0.02 d | 1.29 ± 0.06 d | 1.82 ± 0.02 d |
2.1.1. DPPH Radical Scavenging Activity
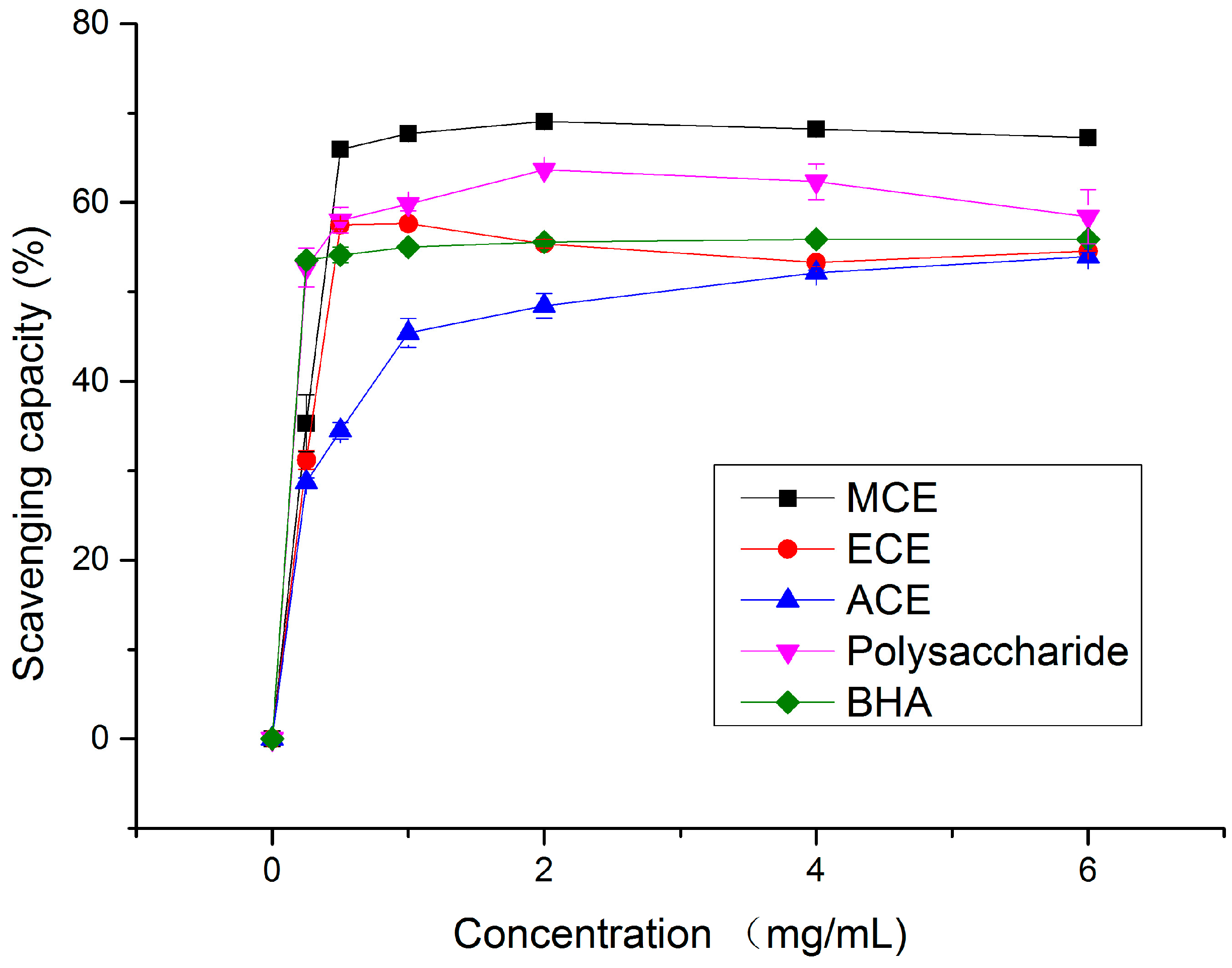
2.1.2. Hydroxy Radical (•OH) Scavenging Activity
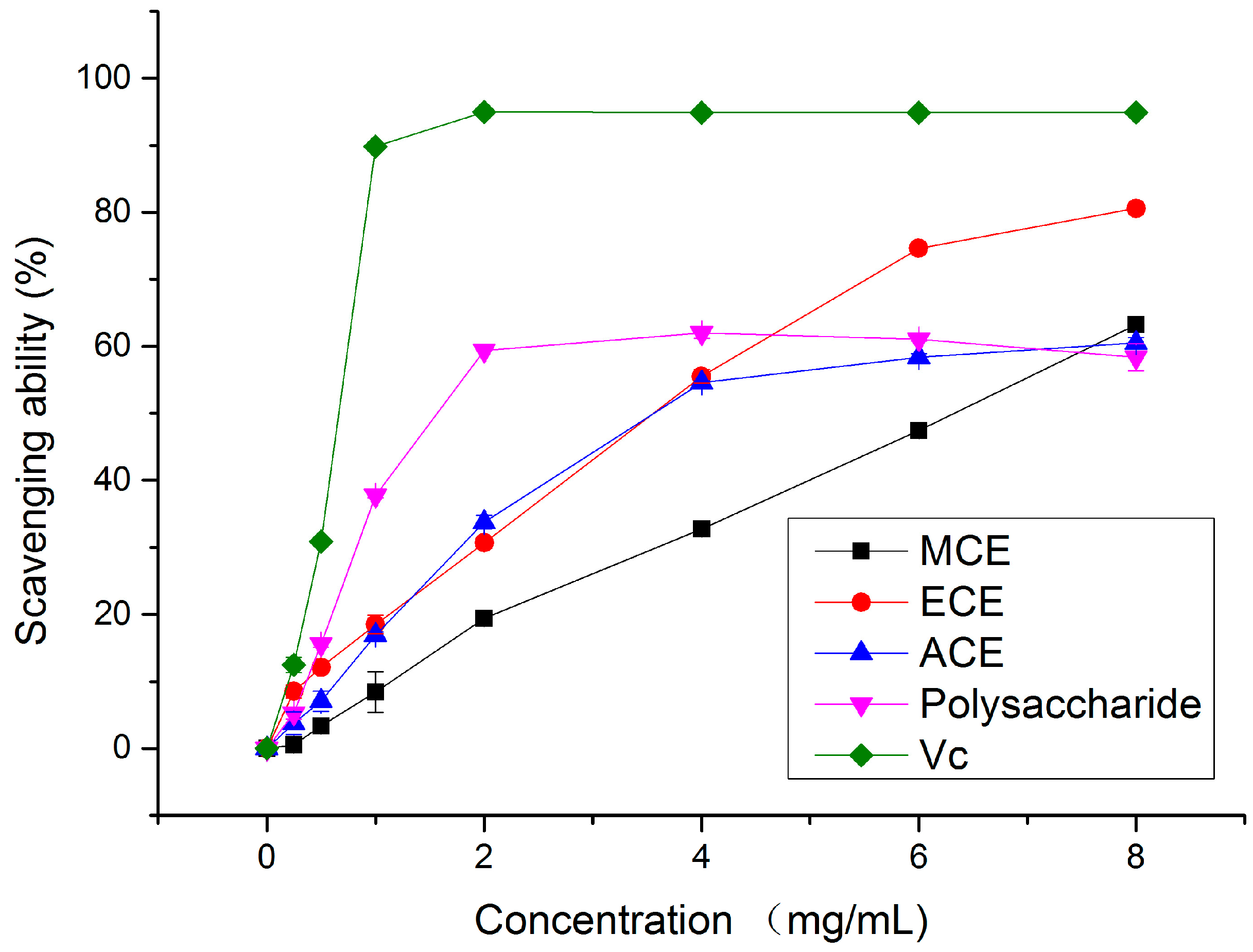
2.1.3. Metal Chelating Activity
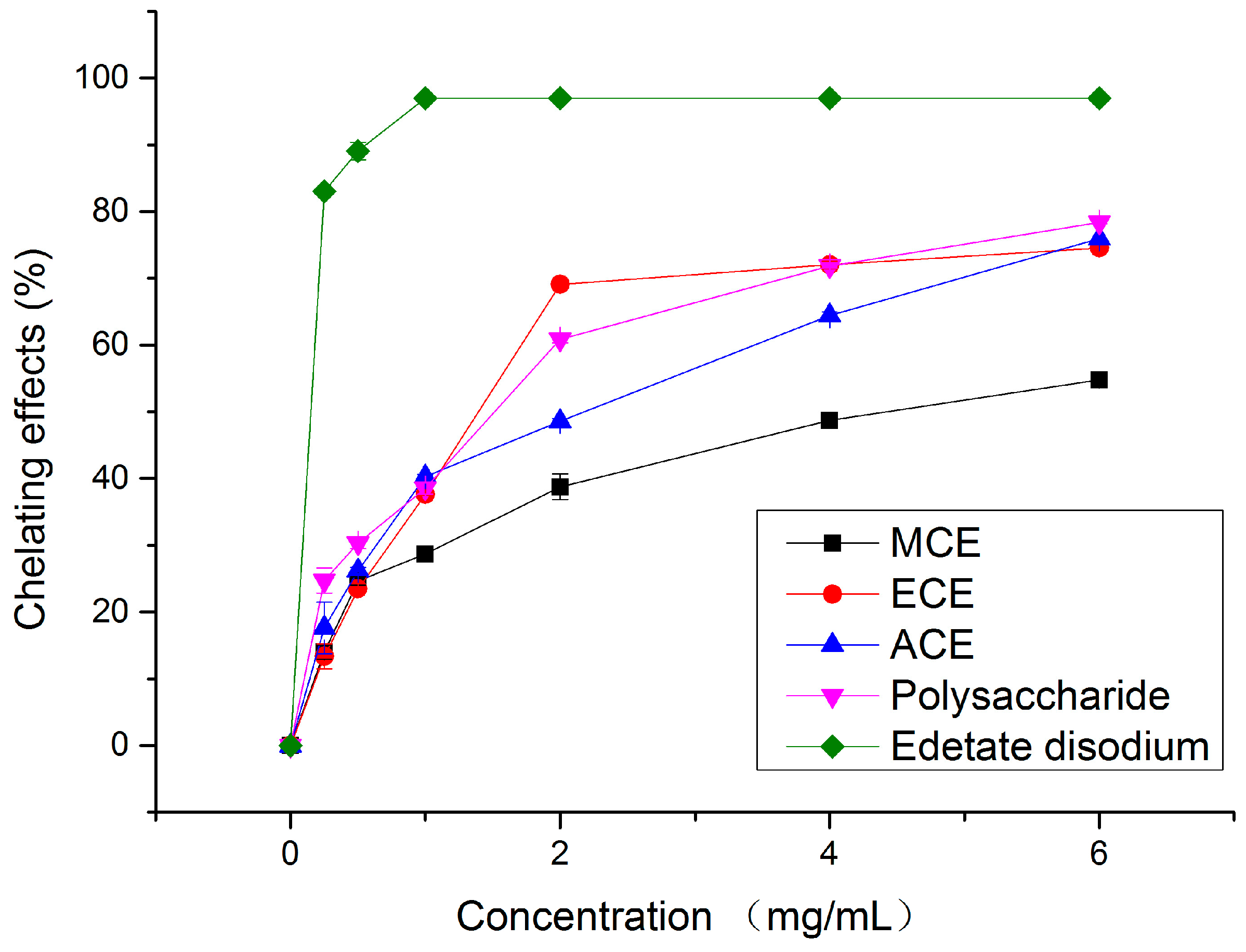
2.1.4. Reducing Power
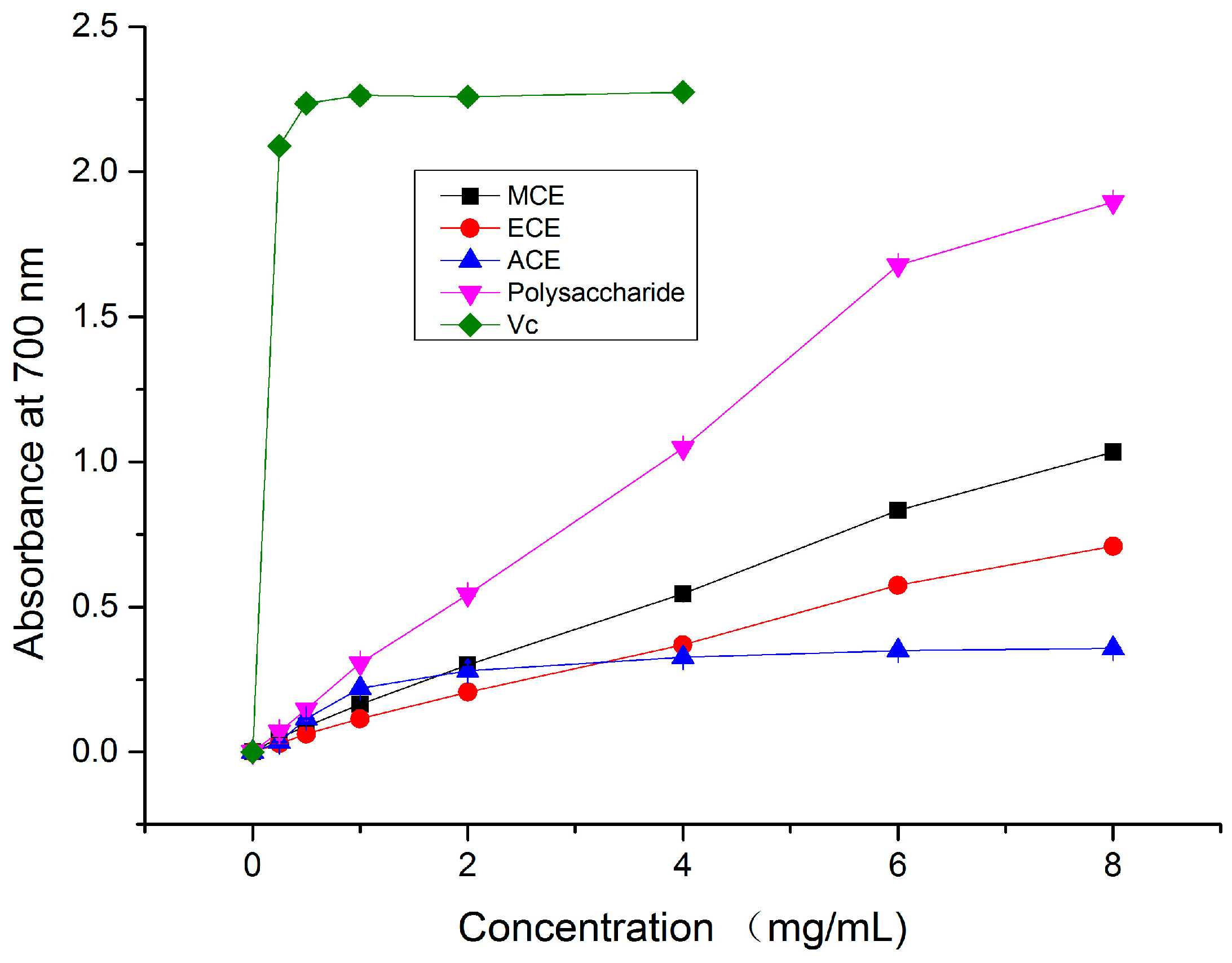
2.2. Anti-Hypoxic Activity
2.2.1. Effect on the Survival Time Test
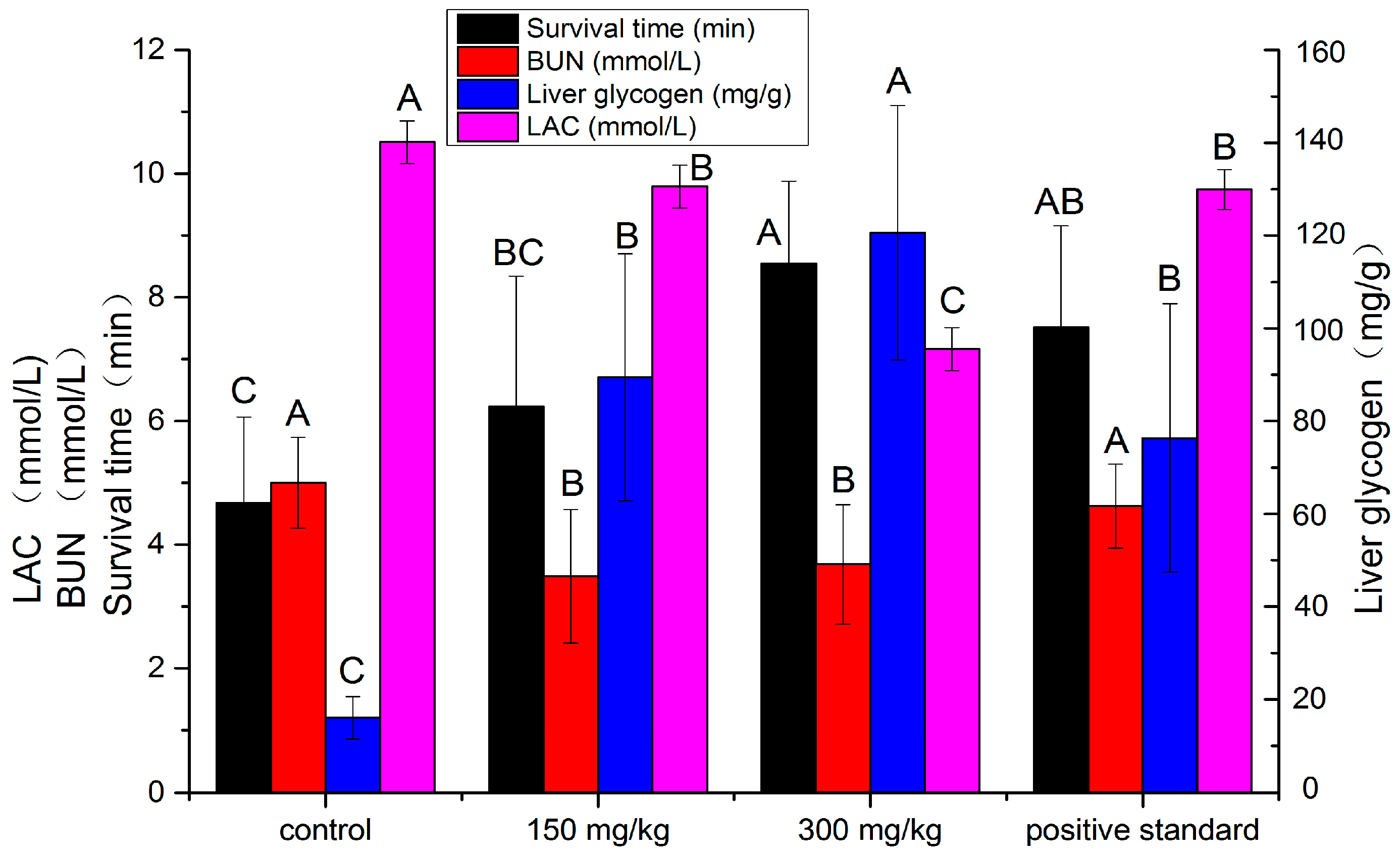
2.2.2. Effect on the Blood Urea Nitrogen Test
2.2.3. Effect on the Liver Glycogen Test
2.2.4. Effect on the Lactic Acid Test
2.3. Molecular Weight and Monosaccharide Compositions
3. Experimental Section
3.1. Sample Preparation
3.2. Extraction
3.3. Antioxidant Activity Assays
3.4. Animal
3.5. Pharmacological Studies
3.5.1. Survival Time Test
3.5.2. Blood Urea Nitrogen (BUN)
3.5.3. Liver Glycogen
3.5.4. Lactic Acid (LAC)
3.6. Isolation, Purification and Identification of Polysaccharide
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Stajic, M.; Vukojevic, J.; Knezevic, A.; Lausevic, S.D.; Milovanovic, I. Antioxidant protective effects of mushroom metabolites. Curr. Top. Med. Chem. 2013, 13, 2660–2676. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen-toxicity, oxygen radicals, transition-metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.; Min, D. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wang, W.-Y.; Wang, X.-L.; Dong, L.-W.; Yue, Y.-T.; Xin, H.-L.; Ling, C.-Q.; Li, M. Anti-hypoxic activity of the ethanol extract from portulaca oleracea in mice. J. Ethnopharmacol. 2009, 124, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C. Acute mountain sickness: Medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad. Med. J. 2006, 82, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-P.; Fan, P.-C.; Jing, L.-L.; Yao, J.; He, X.-R.; Yang, Y.; Chen, K.-M.; Jia, Z.-P. Anti-hypoxic activity at simulated high altitude was isolated in petroleum ether extract of saussurea involucrata. J. Ethnopharmacol. 2011, 137, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured cordyceps mycelia. Phytomedicine 2006, 13, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H.; Xiao, D.M.; Chen, D.X.; Xiao, Y.; Liang, Z.Q.; Zhong, J.J. Polysaccharides from the medicinal mushroom cordyceps taii show antioxidant and immunoenhancing activities in a d-galactose-induced aging mouse model. Evid. Based Complement. Altern. 2012, 2012. [Google Scholar] [CrossRef]
- Xu, T.T.; Beelman, R.B.; Lambert, J.D. The cancer preventive effects of edible mushrooms. Anti-Cancer Agent Med. Chem. 2012, 12, 1255–1263. [Google Scholar] [CrossRef]
- Bai, M.S.; Wang, C.; Zong, S.C.; Lei, M.; Gao, J.M. Antioxidant polyketide phenolic metabolites from the edible mushroom cortinarius purpurascens. Food Chem. 2013, 141, 3424–3427. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, L.N.; Huang, Z.Y.; Su, H.M. Hispidin produced from phellinus linteus protects against peroxynitrite-mediated DNA damage and hydroxyl radical generation. Chem. Biol. Interact. 2012, 199, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Yen, W.J.; Huang, S.C.; Duh, P.D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.Q.; Wang, J.H.; Zha, X.Q.; Zhang, J.B. Structural analysis and antioxidant activities of polysaccharide isolated from jinqian mushroom. Int. J. Biol. Macromol. 2014, 64, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Zhang, Q.B.; Zhao, T.T.; Hu, R.G.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from ulva pertusa (chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (aguricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.A.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, pleurotus ostreatus. Innov. Food Sci. Emerg. 2009, 10, 228–234. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Huang, S.J.; Lo, S.H.; Wu, T.P.; Lian, P.Y.; Mau, J.L. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem. 2009, 113, 578–584. [Google Scholar] [CrossRef]
- Sudha, G.; Vadivukkarasi, S.; Shree, R.B.I.; Lakshmanan, P. Antioxidant activity of various extracts from an edible mushroom pleurotus eous. Food Sci. Biotechnol. 2012, 21, 661–668. [Google Scholar] [CrossRef]
- Wu, G.H.; Hu, T.; Li, Z.Y.; Huang, Z.L.; Jiang, J.G. In vitro antioxidant activities of the polysaccharides from pleurotus tuber-regium (fr.) sing. Food Chem. 2014, 148, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Igarashi, K.; Hirose, S.; Kitagawa, H. Inhibition by polyamines of lipid peroxide formation in rat-liver microsomes. Biochem. Biophs. Res. Commun. 1979, 87, 388–394. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Antioxidative and anti-glycation activity of garcinol from garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms agaricus bisporus, agaricus brasiliensis, ganoderma lucidum and phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Guo, T.; Wei, L.; Sun, J.; Hou, C.L.; Fan, L. Antioxidant activities of extract and fractions from tuber indicum cooke & massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar]
- Witkowska, A.M.; Zujko, M.E.; Mironczuk-Chodakowska, I. Comparative study of wild edible mushrooms as sources of antioxidants. Int. J. Med. Mushrooms 2011, 13, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ciric, A.; van Griensven, L.J.L.D.; Sokovic, M.; Ferreira, I.C.F.R. The methanolic extract of cordyceps militaris (l) link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Paik, I.Y.; Jacobs, K.A. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar] [PubMed]
- Schurr, A. Energy metabolism, stress hormones and neural recovery from cerebral ischemia/hypoxia. Neurochem. Int. 2002, 41, 1–8. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Su, D.; Pi, N.; Ma, C.; Gao, P. Composition analysis and anti-hypoxia activity of polysaccharide from brassica rapa l. Int. J. Biol. Macromol. 2010, 47, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.G.; Jiang, S.P.; Luo, P.; Wu, J.; Gao, P. Isolation, purification and structure identification of antioxidant compound from the roots of incarvillea younghusbandii sprague and its life span prolonging effect in drosophila melanogaster. Nat. Prod. Res. 2008, 22, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the mushroom Agaricus bisporus (Lange) Sing. Chaidam are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-J.; Chen, H.-Y.; Fan, L.-L.; Jiao, Z.-H.; Chen, Q.-H.; Jiao, Y.-C. In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam. Molecules 2015, 20, 17775-17788. https://doi.org/10.3390/molecules201017775
Li H-J, Chen H-Y, Fan L-L, Jiao Z-H, Chen Q-H, Jiao Y-C. In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam. Molecules. 2015; 20(10):17775-17788. https://doi.org/10.3390/molecules201017775
Chicago/Turabian StyleLi, Hong-Ji, Hai-Yan Chen, Lin-Lin Fan, Zhi-Hua Jiao, Qi-He Chen, and Ying-Chun Jiao. 2015. "In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam" Molecules 20, no. 10: 17775-17788. https://doi.org/10.3390/molecules201017775






