The Lectin Frontier Database (LfDB), and Data Generation Based on Frontal Affinity Chromatography
Abstract
:1. Lectins: A Diverse Group of Carbohydrate-Binding Proteins. Investigation and Controversies
2. Trends in Lectin Classification
2.1. Based on Specificity
2.2. Based on Protein Family (Pfam)
3. Determination of Oligosaccharide Specificity of Lectins in Terms of Dissociation Constant (Kd)
3.1. Methods to Determine Kd
- Equilibrium dialysis
- Isothermal calorimetry (ITC)
- Surface plasmon resonance (SPR)
- Fluorescence polarization
- Frontal affinity chromatography (FAC)
- Capillary affinity electrophoresis
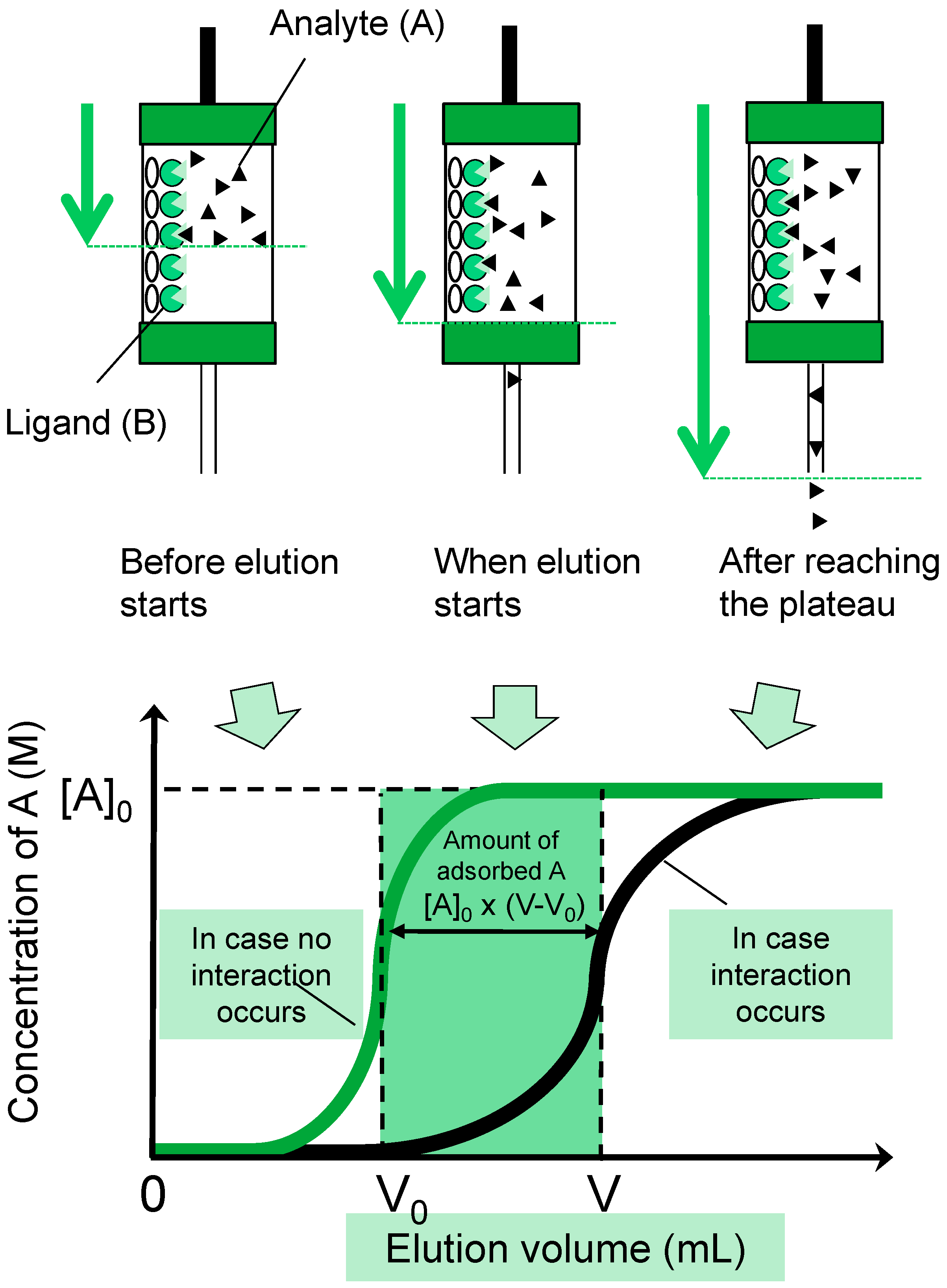
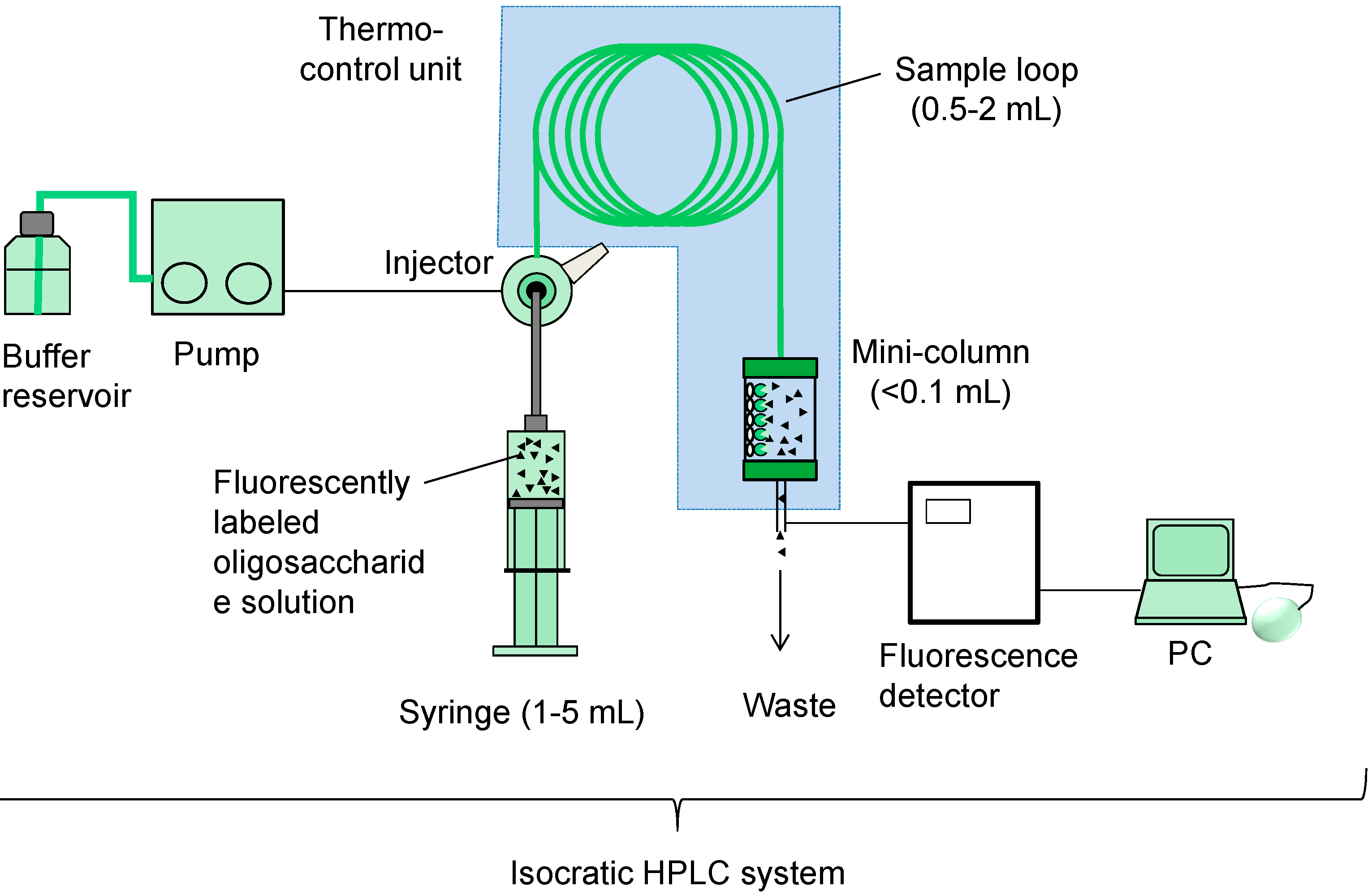
3.2. FAC: Basic Principle and Procedures
| Lectin Categories | Pfam | Protein Fold | Lectins and Special Glycans Analyzed | Ref. |
|---|---|---|---|---|
| L-type (L-type-like) | PF00139 | β-sandwich (jellyroll) | Whisteria (WJA, WFA, WBA) | [55] |
| Pisam sativum (PSL) | [56] | |||
| ERGIC-53, VIPL, VIP36 | [57] | |||
| Erythrina crystagalli (ECL) and other Erythrina species | [58] | |||
| Griffonia simplifolia (GSL-II) | [53] | |||
| Wisteria floribunda (WFA) | [59] | |||
| VIP36 | [60] | |||
| Galectin | PF00337 | β-sandwich (jellyroll) | Mammalian galectins | [61] |
| Fungal galectin (ACG) | [62] | |||
| Mutants of nematode galectin LEC-6 | [63] | |||
| Conger eel (congerin P) | [64] | |||
| Marine sponge Halichondria okadai | [65] | |||
| Nematode gene DC2.3 | [66] | |||
| Mutants of ACG | [67] | |||
| Two lectin domains of nematode galectin LEC-1 | [68] | |||
| Human galectins 1-9 and other lectins | [38] | |||
| Nematode galectins | [69,70] | |||
| American bullfrog Rna catesbeiana | [71] | |||
| Cysteine-less mutant of human galectin-1 | [72] | |||
| Nematode galectin (LEC-6) | [73] | |||
| Nematode galectins (LEC-1~LEC-11) | [74] | |||
| Human galectins | [75] | |||
| Marine sponge Halichondria okadai | [76] | |||
| Mutants of nematode galectin (LEC-1) | [77] | |||
| Human galectin-9 N-terminal CRD | [78] | |||
| The nematode galectin LEC-1 and its mutants | [79] | |||
| Argasid tick Ornithodoros moubata. | [80] | |||
| Xenopus galectin-VIIa | [81] | |||
| Human, chicken, nematode, sponge, fungal galectrins | [36] | |||
| Human galectin-9 | [82] | |||
| Nematode galectin LEC-1 and N- and C-CRDs | [83] | |||
| C-type | PF00059 | C-type α/β-fold | Atlantic salmon serum | [84] |
| calcium-dependent | Sea cucumber lectin CEL-IV | [85] | ||
| DC-SIGN, DC-SIGNR, and LSECtin | [86] | |||
| Langerin to sulfated and mannsylated glycans | [87] | |||
| MGL1, MGL2, and their mutants | [88] | |||
| Atlantic salmon C-type lectin receptor C (SCLRC) | [89] | |||
| Acorn barnacle Megabalanus rosa, Balanus rostatus | [90] | |||
| R-type | PF00652 | β-trefoil | Mutants of earthworm lectin EW29Ch | [91] |
| ricin B chain-related | RCA120/RCA-I | [58] | ||
| Phytopathogenic Sclerotinia sclerotiorum | [92] | |||
| Jacalin-related | PF01419 | β-prism I | Human ZG16 p | [93] |
| Mannose-binding type Jacalin-related lectins | [37] | |||
| Jacalin | [94] | |||
| Castanea crenata agglutinin (CCA) | [95] | |||
| Cycas revoluta leaf lectin (CRLL) | [95] | |||
| GNA-related monocot-type | PF04152 | β-prism II | Two-domain GNA-related lectins | [96] |
| Sea urchin egg | PF02140 | α/β-fold with two long | Shishamo smelt Osmerus (Spirinchus) lanceolatus | [97] |
| lectin (SUEL)-related or Rha-binding | structural loop | Ayu Plecoglossus altivelis | [98] | |
| Pearl shell Pteria penguin | [99] | |||
| P-type | PF02157 | P-type α/β-fold | Mammalian ER lectin XTP3-B | [100] |
| mannose 6-phosphate receptor homology (MRH) | Gene OS-9 (osteosarcoma-9) | [101,102] | ||
| Malectin | PF11721 | β-sandwich (jellyroll) | ER-resident lectin | [103] |
| Fungal fucose-specific | PF07938 | 6-bladed β-propeller | Mushroom Pholiota squarrosa | [104] |
| Aspergillus oryzae | [105] | |||
| AAL-like | ||||
| Fungal fruit body lectin | PF07367 | α/β-sandwich | Agaricus bisporus agglutinin (ABA) | [106] |
| Others | Mussel, Mytilus galloprovincialis | [107] | ||
| orphan or family | Mushroom Hygrophorus russula | [108] | ||
| unidentified | Eggs of Japanese sea hare Aplysia kurodai | [109] | ||
| Feather star Oxycomanthus japonicas | [110] | |||
| Coronate moon turban Turbo (Lunella) coreensis | [111] | |||
| Mushroom Boletus venenatus | [112] | |||
| Red alga Hypnea japonica | [113] | |||
| Pacific annelid Perinereis nuntia ver. vallata | [114] | |||
| Mushroom Boletopsis leucomelas (BLL) | [53,115] |
3.3. Determination of Bt (Effective Ligand Content) in FAC
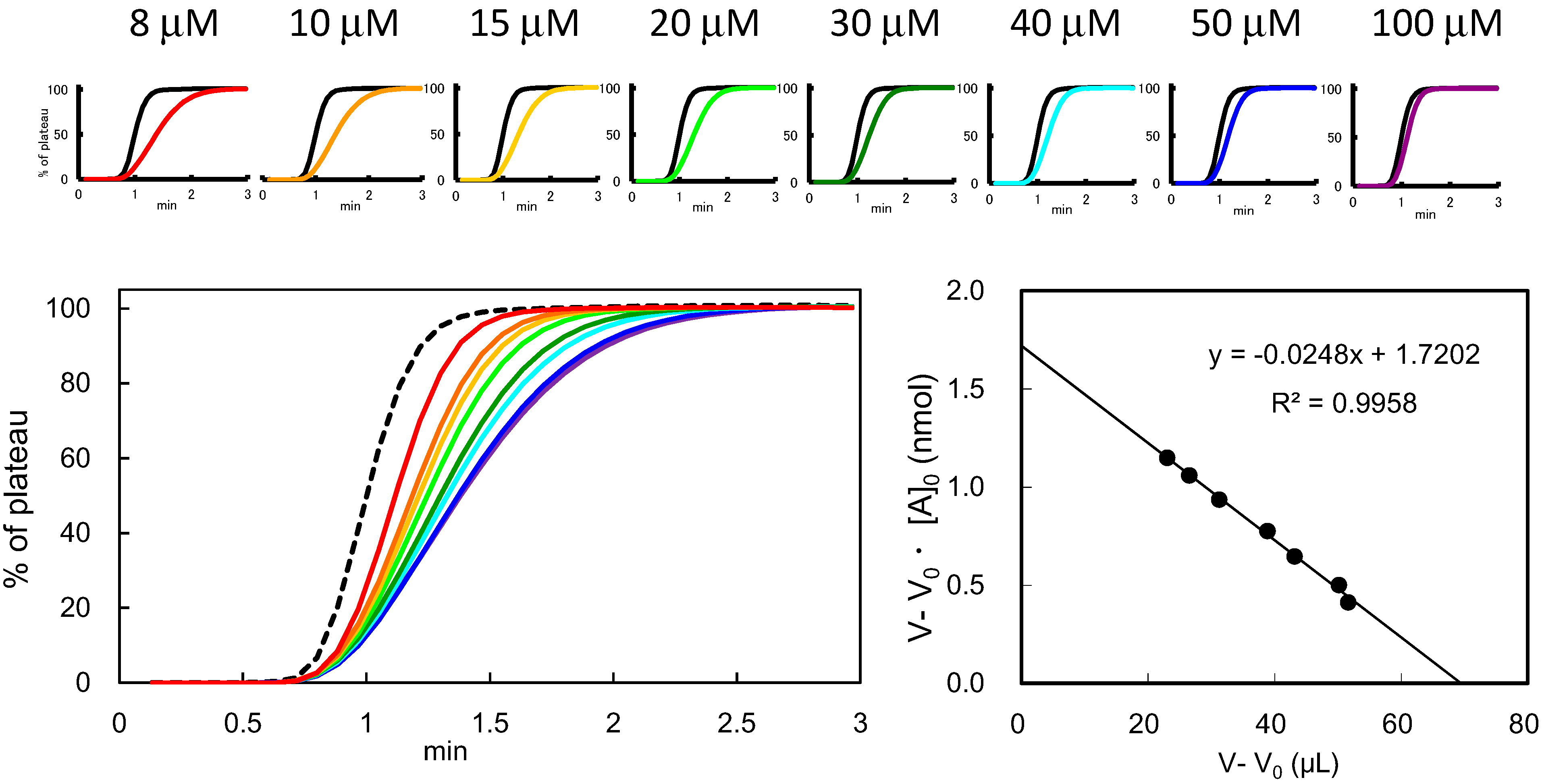
4. Lectin Frontier Database (LfDB)
4.1. Overview of the Database Contents
| Family (Entries) | Pfam ID | Origin | Monosaccharide Specificity (Entries) | FAC Data Deposited |
|---|---|---|---|---|
| Annexin (12) | PF00191 | Mammals, protozoa, etc. | Others (12) | None |
| Chitin-binding (9) | PF00187 | Plants, mammals | GlcNAc (8) | PL/STL, PWM, LEA/LEL, UDA, DSA/DSL, WGA |
| C-type (38) | PF00059 | Mammals, invertebrates | Fuc (5) | None |
| Gal/GalNAc (7) | None | |||
| GlcNAc (3) | None | |||
| Man/Glc (7) | None | |||
| bacteria, virus | Others (2) | None | ||
| Fucose-binding (3) * | PF00754 | Fish | Fuc (1) | None |
| PF07938 | Fungi | Fuc (1) | AAL | |
| PF07472 | Bacteria | Fuc (1) | None | |
| Galectin (26) | PF00337 | Mammals, amphibian, fish, | Gal (23) | Xgalectin VIIa, C14, C16, |
| Invertebrates, fungi | Gal (3) | GC1, GC2, ACG | ||
| Jacalin-related (19) | PF01419 | Plants | Gal (3) | Jacalin |
| Man/Glc (13) | BanLec, Conarva, CRLL, CCA, MornigaM, Calsepa, Heltuba, KM+/Artocarpin | |||
| Legume lectins (37) | PF00139 | Plants | Fuc (2) | LTL, UEA-I |
| Gal/GalNAc (16) | ECA/ECL, EcorL, ECafL, ELysL, EFlaL, DBA/DBL, BPA, BPL, PTL-I, PNA, SBA/SBL, VVL, WFA | |||
| GlcNAc (1) | BSL-II/GSL-II/GS-II | |||
| Man/Glc (9) | PSA/PSL,LCA/LCL/LcH | |||
| Others (2) | PHA-E, PHA-L | |||
| Man-6Pi-binding (3) | PF02157 | Mammals | Man-6Pi (3) | None |
| Monocot (15) | PF01453 | Plants | Man/GlcNAc (11) | HHL, GNA/GNL, NPA/NPL, TxLCI |
| unknown (4) | None | |||
| R-type (19) | PF00652 | Mammals, invertebrates | Gal/GalNAc (11) | RCA-I/RCA120, EW29-Ch |
| Plants, bacteria | NeyAc (4) | SSA, SNA | ||
| Others (4) | None |
4.2. How to Use LfDB
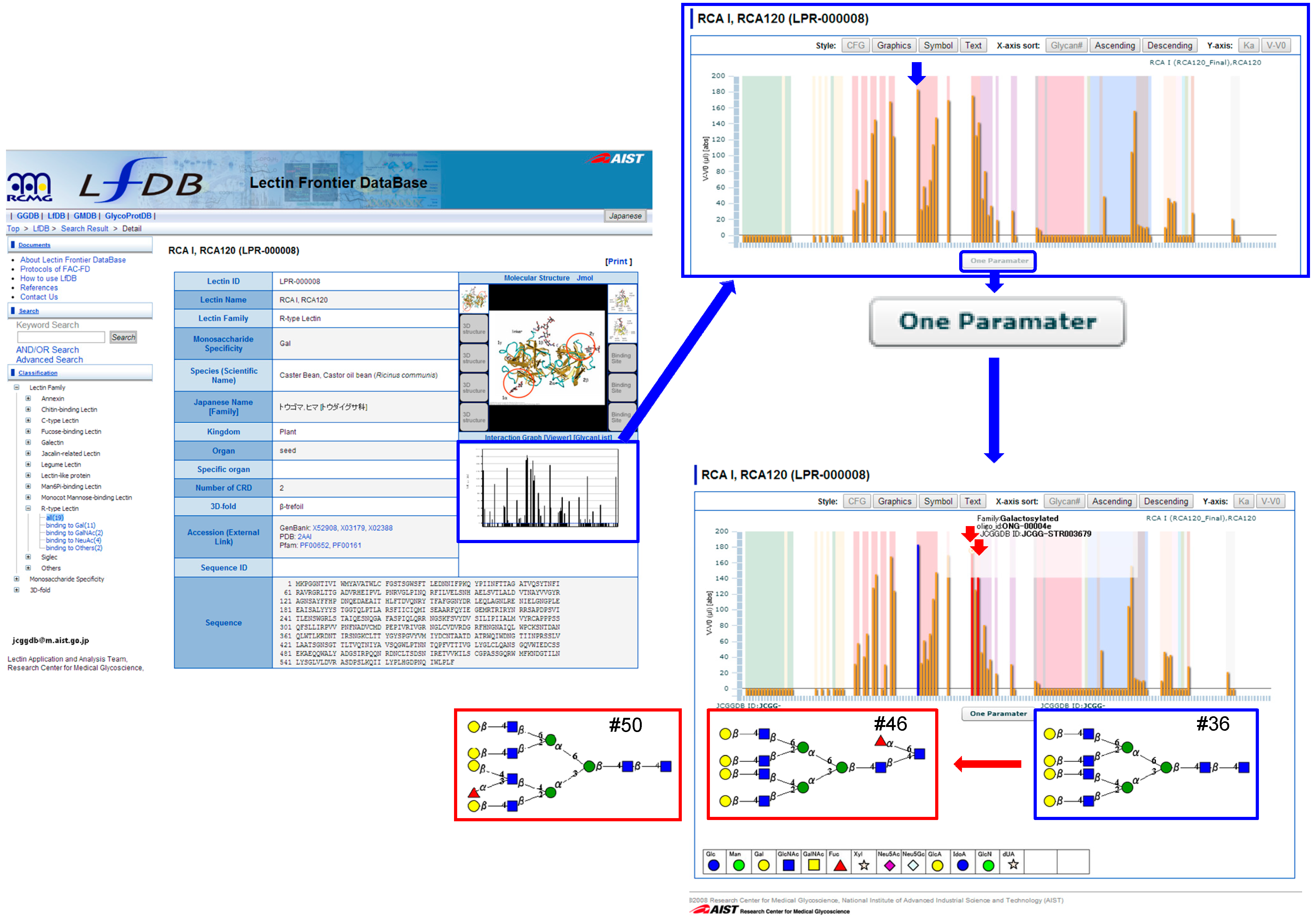
4.3. One-Parameter Difference Analysis
5. Future Plan for Improving the LfDB
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stillmark, H. Über Ricin ein giftiges Ferment aus den Samen von Ricinus communis L. und einige anderen Euphorbiaceen; Inaugural Dissertation Dorpat: Tartu, Russia, 1888. [Google Scholar]
- Van Damme, E.J.M. History of plant lectin research. Methods Mol. Biol. 2014, 1200, 3–13. [Google Scholar]
- Cummings, R.D.; Etzler, M.E. R-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 403–414. [Google Scholar]
- Kilpatrick, D. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar]
- Charcot, J.M.; Robin, C. Observation de leocythemie. Mem. Soc. Biol. 1853, 5, 44–50. [Google Scholar]
- Swaminathan, G.J.; Leonidas, D.D.; Savage, M.P.; Ackerman, S.J.; Acharya, K.R. Selective recognition of mannose by the human eosinophil Charcot-Leyden crystal protein (Galectin 10): A crystallographic study at 1.8 A resolution. Biochemistry 1999, 38, 13837–13843. [Google Scholar]
- Flexner, S.; Noguchi, H. Snake venom in relation to haemolysis, bacteriolysis, and toxicity. J. Exp. Med. 1902, 6, 277–301. [Google Scholar] [CrossRef]
- Kilpatrick, D.C.; Green, C. Lectins as blood typing reagents. In Advances in Lectin Research; Franz, H., Ed.; Ullstein Mosby: Berlin, Germany, 1992; Volume 5, pp. 51–94. [Google Scholar]
- Hirabayashi, J.; Dutta, S.K.; Kasai, K. Novel galactose-binding proteins in Annelida: Characterization of 29-kDa tandem repeat-type lectins from the earthworm Lumbricus terrestris. J. Biol. Chem. 1998, 273, 14450–14460. [Google Scholar] [CrossRef]
- Cummings, R.D.; McEver, R.P. C-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 439–457. [Google Scholar]
- Etzler, M.E.; Surolia, A.; Cummings, R.D. L-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; pp. 415–424. [Google Scholar]
- Varki, A.; Kornfeld, S. P-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 425–437. [Google Scholar]
- Varki, A.; Crocker, P.R. I-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 459–474. [Google Scholar]
- Cummings, R.D.; Liu, F.-T. Galectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 475–487. [Google Scholar]
- Drickamer, K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 1988, 263, 9557–9560. [Google Scholar]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar]
- Sharon, N.; Lis, H. Lectins; Springer Science & Business Media: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Doyle, R.J. Introduction of lectins and their interactions with microorganisms. In Lectin-Microorganism Interactions; Doyle, R.J., Slifkin, M., Eds.; Marcel Decker: New York, NY, USA, 1994; pp. 1–60. [Google Scholar]
- Hirabayashi, J. On the origin of elementary hexoses. Q. Rev. Biol. 1996, 71, 365–380. [Google Scholar] [CrossRef]
- Leffler, H. Introduction to galectins. Trends Glycosci. Glycotechnol. 1997, 9, 9–19. [Google Scholar] [CrossRef]
- Cooper, D.N.; Barondes, S.H. God must love galectins; he made so many of them. Glycobiology 1999, 9, 979–984. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Arata, Y.; Ko, H.; Kasai, K. Galectins from the nematode Caenorhabditis elegans and the glycome project. Trends Glycosci. Glycotechnol. 2001, 13, 533–549. [Google Scholar]
- Park, S.; Gildersleeve, J.C.; Blixt, O.; Shin, I. Carbohydrate microarrays. Chem. Soc. Rev. 2013, 42, 4310–4326. [Google Scholar]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. The Pfam protein families database. Nucl. Acids Res. 2014, 42, D222–D230. [Google Scholar]
- Peumans, W.J.; Van Damme, E.J.; Barre, A.; Rougé, P. Classification of plant lectins in families of structurally and evolutionary related proteins. Adv. Exp. Med. Biol. 2001, 491, 27–54. [Google Scholar]
- Kobayashi, Y.; Tateno, H.; Ogawa, H.; Yamamoto, K.; Hirabayashi, J. Comprehensive list of lectins: Origins, natures, and carbohydrate specificities. Methods Mol. Biol. 2014, 1200, 555–577. [Google Scholar]
- Fujimoto, Z.; Tateno, H.; Hirabayashi, J. Lectin structures: Classification based on the 3-D structures. Methods Mol. Biol. 2014, 1200, 579–606. [Google Scholar]
- Hatakeyama, T. Equilibrium dialysis using chromophoric sugar derivatives. Methods Mol. Biol. 2014, 1200, 165–171. [Google Scholar]
- Takeda, Y.; Matsuo, I. Isothermal calorimetric analysis of lectin-sugar interaction. Methods Mol. Biol. 2014, 1200, 207–214. [Google Scholar]
- Shinohara, Y.; Furukawa, J. Surface plasmon resonance as a tool to characterize lectin-carbohydrate interactions. Methods Mol. Biol. 2014, 1200, 185–205. [Google Scholar]
- Sörme, P.; Kahl-Knutson, B.; Wellmar, U.; Nilsson, U.J.; Leffler, H. Fluorescence polarization to study galectin-ligand interactions. Methods Enzymol. 2003, 362, 504–512. [Google Scholar]
- Kinoshita, M.; Kakehi, K. Capillary-based lectin affinity electrophoresis for interaction analysis between lectins and glycans. Methods Mol. Biol. 2014, 1200, 131–146. [Google Scholar]
- Ohyama, Y.; Kasai, K.; Nomoto, H.; Inoue, Y. Frontal affinity chromatography of ovalbumin glycoasparagines on a concanavalin A-sepharose column. A quantitative study of the binding specificity of the lectin. J. Biol. Chem. 1985, 260, 6882–6887. [Google Scholar]
- Ng, E.S.; Chan, N.W.; Lewis, D.F.; Hindsgaul, O.; Schriemer, D.C. Frontal affinity chromatography-mass spectrometry. Nat. Protoc. 2007, 2, 1907–1917. [Google Scholar]
- Fort, S.; Kim, H.S.; Hindsgaul, O. Screening for galectin-3 inhibitors from synthetic lacto-N-biose libraries using microscale affinity chromatography coupled to mass spectrometry. J. Org. Chem. 2006, 71, 7146–7154. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar]
- Nakamura-Tsuruta, S.; Uchiyama, N.; Peumans, W.J.; Van Damme, E.J.; Totani, K.; Ito, Y.; Hirabayashi, J. Analysis of the sugar-binding specificity of mannose-binding-type Jacalin-related lectins by frontal affinity chromatography: An approach to functional classification. FEBS J. 2008, 275, 1227–1239. [Google Scholar] [CrossRef]
- Iwaki, J.; Tateno, H.; Nishi, N.; Minamisawa, T.; Nakamura-Tsuruta, S.; Itakura, Y.; Kominami, J.; Urashima, T.; Nakamura, T.; Hirabayashi, J. The Galβ-(syn)-gauche configuration is required for galectin-recognition disaccharides. Biochim. Biophys. Acta 2011, 1810, 643–651. [Google Scholar]
- Sato, C. Frontal affinity chromatography: Practice of weak interaction analysis between lectins and fluorescently labeled oligosaccharides. Methods Mol. Biol. 2014, 1200, 257–264. [Google Scholar]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Frontal affinity chromatography: Sugar-protein interactions. Nat. Protoc. 2007, 2, 2529–2537. [Google Scholar] [PubMed]
- Nakamura-Tsuruta, S.; Uchiyama, N.; Kominami, J.; Hirabayashi, J. Frontal affinity chromatography: Systematization for quantitative interaction analysis between lectins and glycans. In Lectins: Analytical Technologies; Nilsson, C.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 239–266. [Google Scholar]
- Gemeiner, P.; Mislovicová, D.; Tkác, J.; Svitel, J.; Pätoprstý, V.; Hrabárová, E.; Kogan, G.; Kozár, T. Lectinomics II: A highway to biomedical/clinical diagnostics. Biotechnol. Adv. 2009, 27, 1–15. [Google Scholar] [CrossRef]
- Gupta, G.; Surolia, A.; Sampathkumar, S.G. Lectin microarrays for glycomic analysis. OMICS 2010, 14, 419–436. [Google Scholar] [CrossRef]
- Hsu, K.L.; Mahal, L.K. Sweet tasting chips: Microarray-based analysis of glycans. Curr. Opin. Chem. Biol. 2009, 13, 427–432. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kuno, A.; Tateno, H. Lectin-based structural glycomics: A practical approach to complex glycans. Electrophoresis 2011, 32, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Yamada, M.; Kuno, A.; Tateno, H. Lectin microarrays: Concept, principle and applications. Chem. Soc. Rev. 2013, 42, 4443–4458. [Google Scholar] [CrossRef]
- Hirabayashi, J. Concept, strategy and realization of lectin-based glycan profiling. J. Biochem. 2008, 144, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Ishii, S. Quantitative analysis of affinity chromatography of trypsin: A new technique for investigation of protein-ligand interaction. J. Biochem. 1975, 77, 261–264. [Google Scholar]
- Kasai, K.; Oda, Y.; Nishikata, M.; Ishii, S. Frontal affinity chromatography: Theory for its application to studies on specific interactions of biomolecules. J. Chromatogr. 1986, 376, 33–47. [Google Scholar] [CrossRef]
- Kasai, K. Frontal affinity chromatography (FAC): Theory and basic aspects. Methods Mol. Biol. 2014, 1200, 243–256. [Google Scholar]
- Kasai, K. Frontal affinity chromatography: A unique research tool for biospecific interaction that promotes glycobiology. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 215–234. [Google Scholar] [CrossRef]
- Hirabayashi, J. Lectin-based structural glycomics: Glycoproteomics and glycan profiling. Glycoconj. J. 2004, 21, 35–40. [Google Scholar] [CrossRef]
- Nakamura-Tsuruta, S.; Kominami, J.; Kamei, M.; Koyama, Y.; Suzuki, T.; Isemura, M.; Hirabayashi, J. Comparative analysis by frontal affinity chromatography of oligosaccharide specificity of GlcNAc-binding lectins, Griffonia simplicifolia lectin-II (GSL-II) and Boletopsis leucomelas lectin (BLL). J. Biochem. 2006, 140, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Butschi, A.; Titz, A.; Wälti, M.A.; Olieric, V.; Paschinger, K.; Nöbauer, K.; Guo, X.; Seeberger, P.H.; Wilson, I.B.; Aebi, M.; et al. Caenorhabditis elegans N-glycan core β-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 2010, 6, e1000717. [Google Scholar]
- Soga, K.; Teruya, F.; Tateno, H.; Hirabayashi, J.; Yamamoto, K. Terminal N-acetylgalactosamine-specific leguminous lectin from Wisteria japonica as a probe for human lung squamous cell carcinoma. PLoS One 2013, 8, e83886. [Google Scholar] [PubMed]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 2009, 19, 527–536. [Google Scholar] [CrossRef]
- Kamiya, Y.; Kamiya, D.; Yamamoto, K.; Nyfeler, B.; Hauri, H.P.; Kato, K. Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL, and VIP36. J. Biol. Chem. 2008, 283, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Nakamura-Tsuruta, S.; Kominami, J.; Sharon, N.; Kasai, K.; Hirabayashi, J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: A search by frontal affinity chromatography. J. Biochem. 2007, 142, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, Y.; Sato, T.; Niwa, T.; Nakamura, S.; Gotoh, M.; Ikehara, S.K.; Kiyohara, K.; Aoki, C.; Iwai, T.; Nakanishi, H.; et al. Apical Golgi localization of N,N'-diacetyllactosediamine synthase, beta4GalNAc-T3, is responsible for LacdiNAc expression on gastric mucosa. Glycobiology 2006, 16, 777–785. [Google Scholar]
- Kamiya, Y.; Yamaguchi, Y.; Takahashi, N.; Arata, Y.; Kasai, K.; Ihara, Y.; Matsuo, I.; Ito, Y.; Yamamoto, K.; Kato, K. Sugar-binding properties of VIP36, an intracellular animal lectin operating as a cargo receptor. J. Biol. Chem. 2005, 280, 37178–37182. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tamura, M.; Nishiyama, K.; Iwaki, J.; Hirabayashi, J.; Takahashi, H.; Natsugari, H.; Arata, Y.; Kasai, K. Mammalian galectins bind Galactoseβ1–4Fucose disaccharide, a unique structural component of protostomial N-type glycoproteins. Biochem. Biophys. Res. Commun. 2013, 436, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Tateno, H.; Sato, T.; Narimatsu, H.; Hirabayashi, J. Tailoring GalNAcα1–3Galβ-specific lectins from a multi-specific fungal galectin: Dramatic change of carbohydrate specificity by a single amino-acid substitution. Biochem. J. 2013, 453, 261–270. [Google Scholar]
- Makyio, H.; Takeuchi, T.; Tamura, M.; Nishiyama, K.; Takahashi, H.; Natsugari, H.; Arata, Y.; Kasai, K.; Yamada, Y.; Wakatsuki, S.; et al. Structural basis of preferential binding of fucose-containing saccharide by the Caenorhabditis elegans galectin LEC-6. Glycobiology 2013, 23, 797–805. [Google Scholar]
- Watanabe, M.; Nakamura, O.; Muramoto, K.; Ogawa, T. Allosteric regulation of the carbohydrate-binding ability of a novel conger eel galectin by D-mannoside. J. Biol. Chem. 2012, 287, 31061–31072. [Google Scholar] [PubMed]
- Matsumoto, R.; Fujii, Y.; Kawsar, S.M.; Kanaly, R.A.; Yasumitsu, H.; Koide, Y.; Hasan, I.; Iwahara, C.; Ogawa, Y.; Im, C.H.; et al. Cytotoxicity and glycan-binding properties of an 18 kDa lectin isolated from the marine sponge Halichondria okadai. Toxins (Basel) 2012, 4, 323–338. [Google Scholar]
- Nemoto-Sasaki, Y.; Takai, S.; Takeuchi, T.; Arata, Y.; Nishiyama, K.; Yamada, A.; Takahashi, H.; Natsugari, H.; Kasai, K. The DC2.3 gene in Caenorhabditis elegans encodes a galectin that recognizes the galactoseβ1→4fucose disaccharide unit. Biol. Pharm. Bull. 2011, 34, 1635–1639. [Google Scholar]
- Imamura, K.; Takeuchi, H.; Yabe, R.; Tateno, H.; Hirabayashi, J. Engineering of the glycan-binding specificity of Agrocybe cylindracea galectin towards α(2,3)-linked sialic acid by saturation mutagenesis. J. Biochem. 2011, 150, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Sugiura, K.; Nishiyama, K.; Takahashi, H.; Natsugari, H.; Arata, Y.; Kasai, K. Sugar-binding properties of the two lectin domains of LEC-1 with respect to the Galβ1–4Fuc disaccharide unit present in protostomia glycoconjugates. Biol. Pharm. Bull. 2011, 34, 1134–1138. [Google Scholar]
- Nishiyama, K.; Yamada, A.; Takahashi, M.; Takeuchi, T.; Kasai, K.; Kobayashi, S.; Natsugari, H.; Takahashi, H. Synthesis of fluorescence-labeled Galbeta1–3Fuc and Galbeta1–4Fuc as probes for the endogenous glyco-epitope recognized by galectins in Caenorhabditis elegans. Chem. Pharm. Bull. (Tokyo) 2010, 58, 495–500. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nishiyama, K.; Sugiura, K.; Takahashi, M.; Yamada, A.; Kobayashi, S.; Takahashi, H.; Natsugari, H.; Kasai, K. Caenorhabditis elegans galectins LEC-6 and LEC-1 recognize a chemically synthesized Galbeta1–4Fuc disaccharide unit which is present in Protostomia glycoconjugates. Glycobiology 2009, 19, 1503–1510. [Google Scholar] [CrossRef]
- Kawsar, S.M.; Matsumoto, R.; Fujii, Y.; Yasumitsu, H.; Uchiyama, H.; Hosono, M.; Nitta, K.; Hamako, J.; Matsui, T.; Kojima, N.; et al. Glycan-binding profile and cell adhesion activity of American bullfrog (Rana catesbeiana) oocyte galectin-1. Protein Pept. Lett. 2009, 16, 677–684. [Google Scholar]
- Nishi, N.; Abe, A.; Iwaki, J.; Yoshida, H.; Itoh, A.; Shoji, H.; Kamitori, S.; Hirabayashi, J.; Nakamura, T. Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology 2008, 18, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Hayama, K.; Hirabayashi, J.; Kasai, K. Caenorhabditis elegans N-glycans containing a Gal-Fuc disaccharide unit linked to the innermost GlcNAc residue are recognized by C. elegans galectin LEC-6. Glycobiology 2008, 18, 882–890. [Google Scholar]
- Nemoto-Sasaki, Y.; Hayama, K.; Ohya, H.; Arata, Y.; Kaneko, M.K.; Saitou, N.; Hirabayashi, J.; Kasai, K. Caenorhabditis elegans galectins LEC-1-LEC-11: Structural features and sugar-binding properties. Biochim. Biophys. Acta 2008, 1780, 1131–1142. [Google Scholar] [PubMed]
- Iwaki, J.; Minamisawa, T.; Tateno, H.; Kominami, J.; Suzuki, K.; Nishi, N.; Nakamura, T.; Hirabayashi, J. Desulfated galactosaminoglycans are potential ligands for galectins: Evidence from frontal affinity chromatography. Biochem. Biophys. Res. Commun. 2008, 373, 206–212. [Google Scholar] [CrossRef]
- Kawsar, S.M.; Fujii, Y.; Matsumoto, R.; Ichikawa, T.; Tateno, H.; Hirabayashi, J.; Yasumitsu, H.; Dogasaki, C.; Hosono, M.; Nitta, K.; et al. Isolation, purification, characterization and glycan-binding profile of a D-galactoside specific lectin from the marine sponge, Halichondria okadai. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 349–357. [Google Scholar]
- Tamura, M.; Kasai, K.; Itagaki, T.; Nonaka, T.; Arata, Y. Identification of a second, non-conserved amino acid that contributes to the unique sugar binding properties of the nematode galectin LEC-1. Biol. Pharm. Bull. 2008, 31, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Nishi, N.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Wakatsuki, S.; Kato, R. Structural analysis of the human galectin-9 N-terminal carbohydrate recognition domain reveals unexpected properties that differ from the mouse orthologue. J. Mol. Biol. 2008, 375, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Arata, Y.; Ishii, N.; Tamura, M.; Nonaka, T.; Kasai, K. Identification of the amino acid residue in the nematode galectin LEC-1 responsible for its unique sugar binding property: Analysis by combination of site-directed mutagenesis and frontal affinity chromatography. Biol. Pharm. Bull. 2007, 30, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tsuji, N.; Miyoshi, T.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Fujisaki, K. Molecular characterization and oligosaccharide-binding properties of a galectin from the argasid tick Ornithodoros moubata. Glycobiology 2007, 17, 313–323. [Google Scholar] [PubMed]
- Shoji, H.; Ikenaka, K.; Nakakita, S.; Hayama, K.; Hirabayashi, J.; Arata, Y.; Kasai, K.; Nishi, N.; Nakamura, T. Xenopus galectin-VIIa binds N-glycans of members of the cortical granule lectin family (xCGL and xCGL2). Glycobiology 2005, 15, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nishi, N.; Shoji, H.; Seki, M.; Hashidate, T.; Hirabayashi, J.; Kasai, K.; Hata, Y.; Suzuki, S.; Hirashima, M.; et al. Functional analysis of the carbohydrate recognition domains and a linker peptide of galectin-9 as to eosinophil chemoattractant activity. Glycobiology 2002, 12, 191–197. [Google Scholar]
- Arata, Y.; Hirabayashi, J.; Kasai, K. Sugar binding properties of the two lectin domains of the tandem repeat-type galectin LEC-1 (N32) of Caenorhabditis elegans: Detailed analysis by an improved frontal affinity chromatography method. J. Biol. Chem. 2001, 276, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Uribe, E.; Steele, T.J.; Richards, R.C.; Ewart, K.V. Ligand and pathogen specificity of the Atlantic salmon serum C-type lectin. Biochim. Biophys. Acta 2013, 1830, 2129–2138. [Google Scholar] [PubMed]
- Hatakeyama, T.; Kamiya, T.; Kusunoki, M.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Goda, S.; Unno, H. Galactose recognition by a tetrameric C-type lectin, CEL-IV, containing the EPN carbohydrate recognition motif. J. Biol. Chem. 2011, 286, 10305–10315. [Google Scholar] [CrossRef] [PubMed]
- Yabe, R.; Tateno, H.; Hirabayashi, J. Frontal affinity chromatography analysis of constructs of DC-SIGN, DC-SIGNR and LSECtin extend evidence for affinity to agalactosylated N-glycans. FEBS J. 2010, 277, 4010–4026. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Ohnishi, K.; Yabe, R.; Hayatsu, N.; Sato, T.; Takeya, M.; Narimatsu, H.; Hirabayashi, J. Dual specificity of Langerin to sulfated and mannosylated glycans via a single C-type carbohydrate recognition domain. J. Biol. Chem. 2010, 285, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Oo-Puthinan, S.; Maenuma, K.; Sakakura, M.; Denda-Nagai, K.; Tsuiji, M.; Shimada, I.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Bovin, N.V.; Irimura, T. The amino acids involved in the distinct carbohydrate specificities between macrophage galactose-type C-type lectins 1 and 2 (CD301a and b) of mice. Biochim. Biophys. Acta 2008, 1780, 89–100. [Google Scholar] [PubMed]
- Soanes, K.H.; Ewart, K.V.; Mattatall, N.R. Recombinant production and characterization of the carbohydrate recognition domain from Atlantic salmon C-type lectin receptor C (SCLRC). Protein Expr. Purif. 2008, 59, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Jimbo, M.; Kamiya, H.; Ogawa, T.; Muramoto, K. Diverse sugar-binding specificities of marine invertebrate C-type lectins. Biosci. Biotechnol. Biochem. 2007, 71, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Tateno, H.; Kuno, A.; Yabe, R.; Hirabayashi, J. Directed evolution of lectins with sugar-binding specificity for 6-sulfo-galactose. J. Biol. Chem. 2012, 287, 20313–20320. [Google Scholar] [PubMed]
- Van Damme, E.J.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Rougé, P.; Peumans, W.J. The Sclerotinia sclerotiorum agglutinin represents a novel family of fungal lectins remotely related to the Clostridium botulinum non-toxin haemagglutinin HA33/A. Glycoconj. J. 2007, 24, 143–156. [Google Scholar] [CrossRef]
- Tateno, H.; Yabe, R.; Sato, T.; Shibazaki, A.; Shikanai, T.; Gonoi, T.; Narimatsu, H.; Hirabayashi, J. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology 2012, 22, 210–220. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, S.; Wang, H.; Iwasaki, H.; Tachibana, K.; Maebara, K.; Cheng, L.; Hirabayashi, J.; Narimatsu, H. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology 2006, 16, 46–53. [Google Scholar] [PubMed]
- Nakamura, S.; Yagi, F.; Totani, K.; Ito, Y.; Hirabayashi, J. Comparative analysis of carbohydrate-binding properties of two tandem repeat-type Jacalin-related lectins, Castanea crenata agglutinin and Cycas revoluta leaf lectin. FEBS J. 2005, 272, 2784–2799. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Nakamura-Tsuruta, S.; Smith, D.F.; Ongenaert, M.; Winter, H.C.; Rougé, P.; Goldstein, I.J.; Mo, H.; Kominami, J.; Culerrier, R.; et al. Phylogenetic and specificity studies of two-domain GNA-related lectins: Generation of multispecificity through domain duplication and divergent evolution. Biochem. J. 2007, 404, 51–61. [Google Scholar]
- Hosono, M.; Sugawara, S.; Tatsuta, T.; Hikita, T.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Kawsar, S.M.; Ozeki, Y.; Hakomori, S.I.; et al. Domain composition of rhamnose-binding lectin from shishamo smelt eggs and its carbohydrate-binding profiles. Fish Physiol. Biochem. 2013, 39, 1619–1630. [Google Scholar]
- Watanabe, Y.; Shiina, N.; Shinozaki, F.; Yokoyama, H.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Sugahara, K.; Kamiya, H.; Matsubara, H.; et al. Isolation and characterization of l-rhamnose-binding lectin, which binds to microsporidian Glugea plecoglossi, from ayu (Plecoglossus altivelis) eggs. Dev. Comp. Immunol. 2008, 32, 487–499. [Google Scholar]
- Naganuma, T.; Ogawa, T.; Hirabayashi, J.; Kasai, K.; Kamiya, H.; Muramoto, K. Isolation, characterization and molecular evolution of a novel pearl shell lectin from a marine bivalve, Pteria penguin. Mol. Divers. 2006, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Kamiya, Y.; Nagata, K.; Kato, K.; Hosokawa, N. Endoplasmic reticulum lectin XTP3-B inhibits endoplasmic reticulum-associated degradation of a misfolded α1-antitrypsin variant. FEBS J. 2013, 280, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Yamaguchi, D.; Tateno, H.; Hu, D.; Qin, S.Y.; Kawasaki, N.; Yamada, M.; Matsumoto, N.; Hirabayashi, J.; Ito, Y.; et al. The sugar-binding ability of human OS-9 and its involvement in ER-associated degradation. Glycobiology 2010, 20, 310–321. [Google Scholar]
- Hosokawa, N.; Kamiya, Y.; Kamiya, D.; Kato, K.; Nagata, K. Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans. J. Biol. Chem. 2009, 284, 17061–17068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, D.; Yabe, R.; Tateno, H.; Qin, S.Y.; Matsumoto, N.; Hirabayashi, J.; Yamamoto, K. Role of malectin in Glc(2)Man(9)GlcNAc(2)-dependent quality control of α1-antitrypsin. Mol. Biol. Cell 2011, 22, 3559–3570. [Google Scholar]
- Kobayashi, Y.; Tateno, H.; Dohra, H.; Moriwaki, K.; Miyoshi, E.; Hirabayashi, J.; Kawagishi, H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 2012, 287, 33973–33982. [Google Scholar] [PubMed]
- Matsumura, K.; Higashida, K.; Hata, Y.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal. Biochem. 2009, 386, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Tsuruta, S.; Kominami, J.; Kuno, A.; Hirabayashi, J. Evidence that Agaricus bisporus agglutinin (ABA) has dual sugar-binding specificity. Biochem. Biophys. Res. Commun. 2006, 347, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Dohmae, N.; Takio, K.; Kawsar, S.M.; Matsumoto, R.; Hasan, I.; Koide, Y.; Kanaly, R.A.; Yasumitsu, H.; Ogawa, Y.; et al. A lectin from the mussel Mytilus galloprovincialis has a highly novel primary structure and induces glycan-mediated cytotoxicity of globotriaosylceramide-expressing lymphoma cells. J. Biol. Chem. 2012, 287, 44772–44783. [Google Scholar]
- Suzuki, T.; Sugiyama, K.; Hirai, H.; Ito, H.; Morita, T.; Dohra, H.; Murata, T.; Usui, T.; Tateno, H.; Hirabayashi, J.; et al. Mannose-specific lectin from the mushroom Hygrophorus russula. Glycobiology 2012, 22, 16–29. [Google Scholar]
- Kawsar, S.M.; Matsumoto, R.; Fujii, Y.; Matsuoka, H.; Masuda, N.; Chihiro, I.; Yasumitsu, H.; Kanaly, R.A.; Sugawara, S.; Hosono, M.; et al. Cytotoxicity and glycan-binding profile of a d-galactose-binding lectin from the eggs of a Japanese sea hare (Aplysia kurodai). Protein J. 2011, 30, 509–519. [Google Scholar]
- Matsumoto, R.; Shibata, T.F.; Kohtsuka, H.; Sekifuji, M.; Sugii, N.; Nakajima, H.; Kojima, N.; Fujii, Y.; Kawsar, S.M.; Yasumitsu, H.; et al. Glycomics of a novel type-2 N-acetyllactosamine-specific lectin purified from the feather star, Oxycomanthus japonicus (Pelmatozoa: Crinoidea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 266–273. [Google Scholar]
- Fujii, Y.; Kawsar, S.M.; Matsumoto, R.; Yasumitsu, H.; Ishizaki, N.; Dogasaki, C.; Hosono, M.; Nitta, K.; Hamako, J.; Taei, M.; et al. A D-galactose-binding lectin purified from coronate moon turban, Turbo (Lunella) coreensis, with a unique amino acid sequence and the ability to recognize lacto-series glycosphingolipids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 30–37. [Google Scholar]
- Horibe, M.; Kobayashi, Y.; Dohra, H.; Morita, T.; Murata, T.; Usui, T.; Nakamura-Tsuruta, S.; Kamei, M.; Hirabayashi, J.; Matsuura, M.; et al. Toxic isolectins from the mushroom Boletus venenatus. Phytochemistry 2010, 71, 648–657. [Google Scholar]
- Okuyama, S.; Nakamura-Tsuruta, S.; Tateno, H.; Hirabayashi, J.; Matsubara, K.; Hori, K. Strict binding specificity of small-sized lectins from the red alga Hypnea japonica for core (α1–6) fucosylated N-glycans. Biosci. Biotechnol. Biochem. 2009, 73, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, S.M.; Takeuchi, T.; Kasai, K.; Fujii, Y.; Matsumoto, R.; Yasumitsu, H.; Ozeki, Y. Glycan-binding profile of a D-galactose binding lectin purified from the annelid, Perinereis nuntia ver. vallata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 382–389. [Google Scholar]
- Koyama, Y.; Suzuki, T.; Odani, S.; Nakamura, S.; Kominami, J.; Hirabayashi, J.; Isemura, M. Carbohydrate specificity of lectins from Boletopsis leucomelas and Aralia cordate. Biosci. Biotechnol. Biochem. 2006, 70, 542–545. [Google Scholar] [PubMed]
- Aoki-Kinoshita, K.F.; Bolleman, J.; Campbell, M.P.; Kawano, S.; Kim, J.D.; Lütteke, T.; Matsubara, M.; Okuda, S.; Ranzinger, R.; Sawaki, H.; et al. Introducing glycomics data into the Semantic Web. J. Biomed. Semant. 2013, 4, 39. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirabayashi, J.; Tateno, H.; Shikanai, T.; Aoki-Kinoshita, K.F.; Narimatsu, H. The Lectin Frontier Database (LfDB), and Data Generation Based on Frontal Affinity Chromatography. Molecules 2015, 20, 951-973. https://doi.org/10.3390/molecules20010951
Hirabayashi J, Tateno H, Shikanai T, Aoki-Kinoshita KF, Narimatsu H. The Lectin Frontier Database (LfDB), and Data Generation Based on Frontal Affinity Chromatography. Molecules. 2015; 20(1):951-973. https://doi.org/10.3390/molecules20010951
Chicago/Turabian StyleHirabayashi, Jun, Hiroaki Tateno, Toshihide Shikanai, Kiyoko F. Aoki-Kinoshita, and Hisashi Narimatsu. 2015. "The Lectin Frontier Database (LfDB), and Data Generation Based on Frontal Affinity Chromatography" Molecules 20, no. 1: 951-973. https://doi.org/10.3390/molecules20010951





