Synthesis of some new Thieno[2,3-b]pyridines, Pyrimidino[4',5':4,5]thieno[2,3-b]pyridine and Pyridines Incorporating 5-Bromobenzofuran-2-yl Moiety
Abstract
:1. Introduction
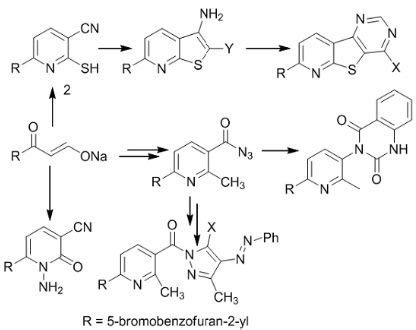
2. Results and Discussion
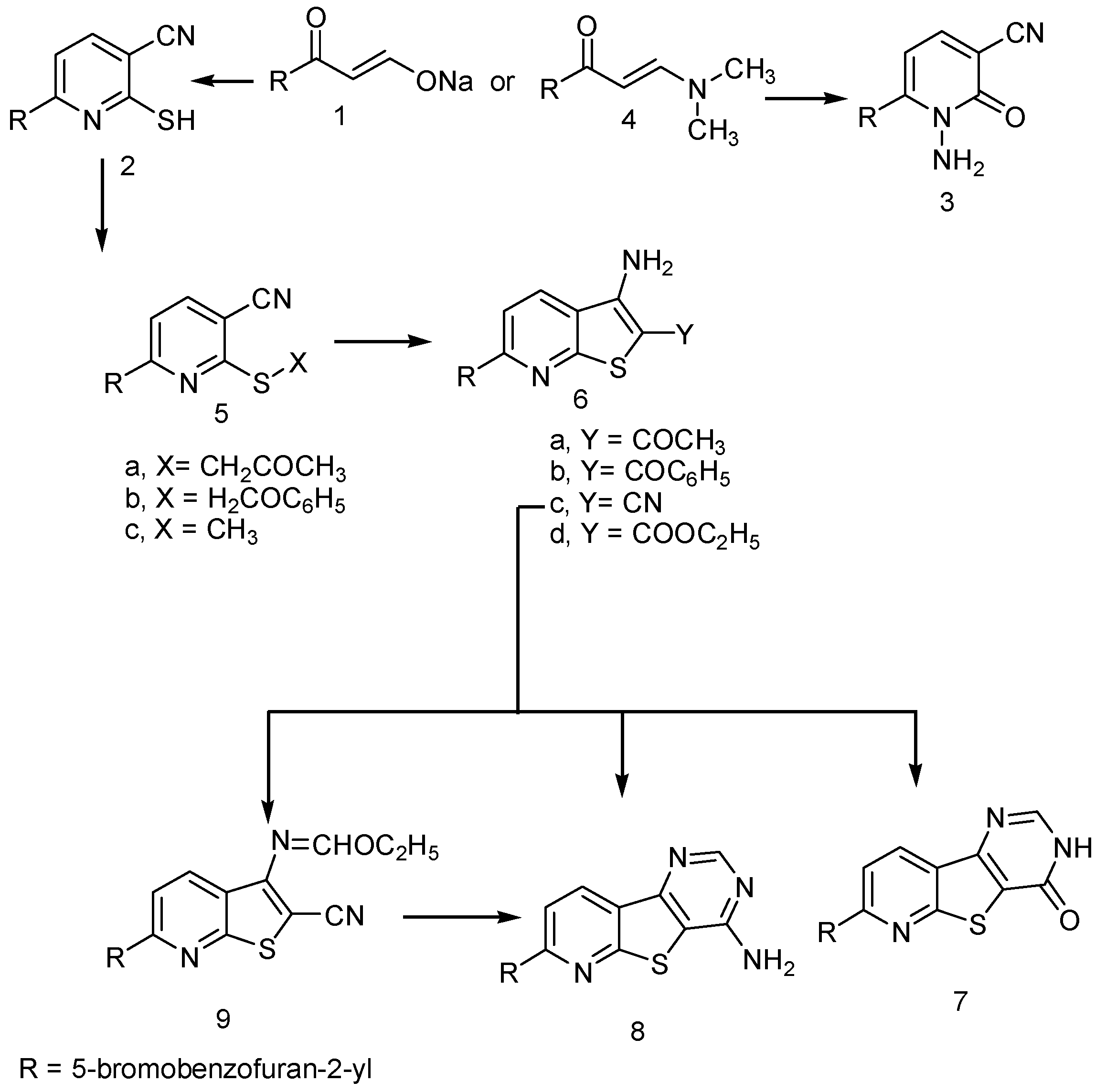
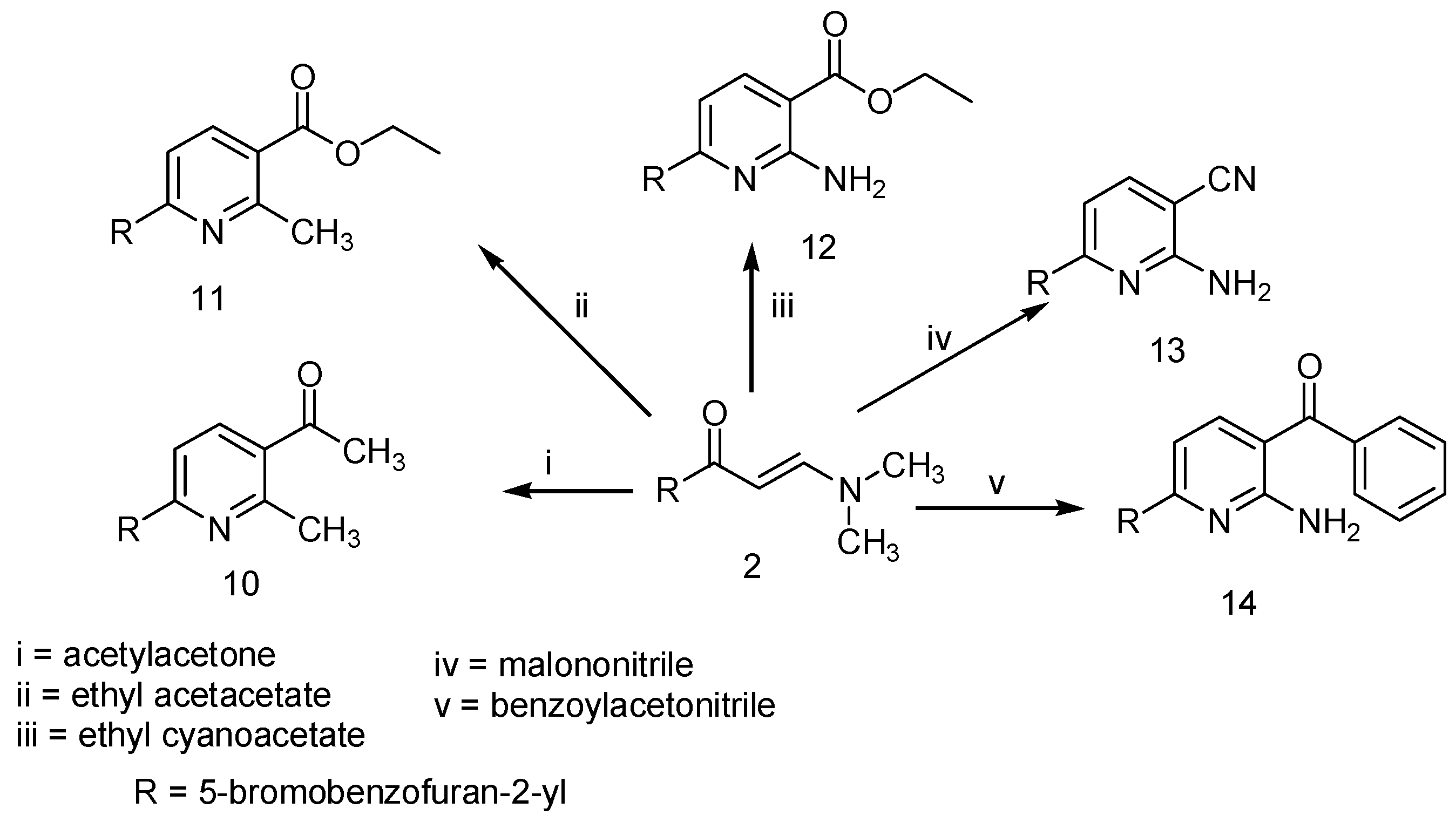

3. Experimental Section
3.1. General Procedure for the Synthesis of 6-(5-Bromobenzofuran-2-yl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (2) and 1-Amino-6-(5-bromobenzofuran-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (3)
3.2. General Procedure for the Synthesis of 6-(5-bromobenzofuran-2-yl)-2-((2-oxopropyl)thio)-nicotinonitrile (5a), 6-(5-bromobenzofuran-2-yl)-2-(2-oxo-2-phenyl-ethylsulfanyl)-nicotinonitrile (5b) and 6-(5-bromobenzofuran-2-yl)-2-(methylthio)nicotinonitrile (5c)
3.3. General Procedure for the Synthesis of 1-(3-amino-6-(5-bromobenzofuran-2-yl)thieno[2,3-b]pyridin-2-yl)ethan-1-one (6a), (3-amino-6-(5-bromobenzofuran-2-yl)thieno[2,3-b]pyridin-2-yl)(phenyl)methanone (6b), 3-amino-6-(5-bromobenzofuran-2-yl)thieno[2,3-b]pyridine-2-carbonitrile (6c) and ethyl 3-Amino-6-(5-bromobenzofuran-2-yl)thieno[2,3-b]pyridine-2-carboxylate (6d)
3.4. Synthesis of 7-(5-bromobenzofuran-2-yl)pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4(3H)-one (7), 7-(5-bromobenzofuran-2-yl)pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-amine (8) and ethyl (E)-N-(6-(5-Bromobenzofuran-2-yl)-2-cyanothieno[2,3-b]pyridin-3-yl)formimidate (9)
3.5. Pyridine Derivatives 10–14
3.6. 1-(6-(5-Bromobenzofuran-2-yl)-2-methylnicotinoyl)-3-methyl-1H-pyrazol-5(4H)-one (16a) and (6-(5-Bromobenzofuran-2-yl)-2-methylpyridin-3-yl)(3,5-dimethyl-1H-pyrazol-1-yl)methanone (16b)
3.7. 2-[6-(5-Bromobenzofuran-2-yl)-2-methyl-pyridine-3-carbonyl]-5-methyl-4-(phenyl-hydrazono)-2,4-dihydro-pyrazol-3-one (17) and (6-(5-bromobenzofuran-2-yl)-2-methylpyridin-3-yl)(3,5-dimethyl-4-(2-phenylhydrazinyl)-1H-pyrazol-1-yl)methanone (18)
3.8. Urea Derivatives 21a–e
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Wu, J.P.; Fleck, R.; Brickwood, J.; Capolino, A.; Catron, K. The discovery of thienopyridine analogues as potent Ikappa B kinase beta inhibitors. Part II. Bioorg. Med. Chem. Lett. 2009, 19, 5547–5551. [Google Scholar]
- Willemann, C.; Grunert, R.; Bednarski, P.J.; Troschutz, R. Synthesis and cytotoxic activity of 5,6-heteroaromatically annulated pyridine-2,4-diamines. Bioorg. Med. Chem. 2009, 17, 4406–4419. [Google Scholar]
- Lockman, J.W.; Reeder, M.D.; Suzuki, K.; Ostanin, K.; Hoff, R.; Bhoite, L.; Austin, H.; Baichwal, V.; Adam, W.J. Inhibition of eEF2-K by thieno[2,3-b]pyridine analogues. Bioorg. Med. Chem. Lett. 2010, 20, 2283–2286. [Google Scholar]
- Zeng, X.X.; Zheng, R.L.; Zhou, T.; He, H.Y.; Liu, J.Y.; Zheng, Y.; Tong, A.P.; Xiang, M.L.; Song, X.R.; Yang, S.Y.; et al. Novel thienopyridine derivatives as specific anti-hepatocellular carcinoma (HCC) agents: synthesis, preliminary structure-activity relationships, and in vitro biological evaluation. Bioorg. Med. Chem. Lett. 2010, 20, 6282–6285. [Google Scholar]
- Pevet, I.; Brule, C.; Tizot, A.; Gohier, A.; Cruzalegui, F.; Boutin, J.A.; Goldstein, S. Synthesis and pharmacological evaluation of thieno[2,3-b]pyridine derivatives as novel c-Src inhibitors. Bioorg. Med. Chem. 2011, 19, 2517–2528. [Google Scholar]
- Mohareb, R.M.; Wardakhan, W.W.; Elmegeed, G.A.; Ashour, R.M. Heterocyclizations of pregnenolone: Novel synthesis of thiosemicarbazone, thiophene, thiazole, thieno[2,3-b]pyridine derivatives and their cytotoxicity evaluations. Steroids 2012, 77, 1560–1569. [Google Scholar]
- Feng, L.; Reynisdóttir, I.; Reynisson, J. The effect of PLC-g2 inhibitors on the growth of human tumour cells. Eur. J. Med. Chem. 2012, 54, 463–469. [Google Scholar]
- Dai, X.Y.; Zeng, X.X.; Peng, F.; Han, Y.Y.; Lin, H.J.; Xu., Y.Z.; Zhou, T.; Xie, G.; Deng, Y.; Mao, Y.Q.; et al. A novel anticancer agent, SKLB70359, inhibits human hepatic carcinoma cells proliferation via G0/G1 cell cycle arrest and apoptosis induction. Cell Physiol. Biochem. 2012, 29, 281–290. [Google Scholar]
- Mohareb, R.M.; Abdallah, A.E.; Abdelaziz, M.A. New approaches for the synthesis of pyrazole, thiophene, thieno[2,3-b]pyridine and thiazole derivatives together with their anti-tumor evaluations. Med. Chem. Res. 2013, 22, 1–13. [Google Scholar]
- Schnute, M.E.; Anderson, D.J.; Brideau, R.J.; Ciske, F.L.; Collier, S.A. 2-Aryl-2-hydroxyethylamine substituted 4-oxo-4,7-dihydrothieno[2,3-b]pyridines as broad-spectrum inhibitors of human herpesvirus polymerases. Bioorg. Med. Chem. Lett. 2007, 17, 3349–3353. [Google Scholar]
- Pinheiro, L.C.S.; Borges, J.C.; Oliveira, C.D.; Ferreira, V.F.; Romeiro, G.A. Synthesis of new (phenylamino)thieno [2,3-b]pyridines and derivatives of the novel benzo[b]thieno[3,2-h]-1,6-naphthyridinetetracyclic system. ARKIVOC 2008, 17, 77–87. [Google Scholar]
- Shuck-Lee, D.; Chen, F.F.; Willard, R.; Raman, S.; Ptak, R. Heterocyclic Compounds that Inhibit Rev-RRE Function and Human Immunodeficiency Virus Type 1 Replication. Antimicrob. Agents Chemother. 2008, 52, 3169–3179. [Google Scholar]
- Chaubey, A.; Pandeya, S.N. Pyridine a versatile nucleuse in pharmaceutical field. Asian J. Pharm. Clin. Res. 2011, 4, 5–8. [Google Scholar]
- Boschelli, D.H.; Wu, B.; Barrios Sosa, A.C.; Chen, J.; Asselin, M.; Cole, D.C.; Lee, J.; Yang, X.; Chaudhary, D. Synthesis and PKCtheta inhibitory activity of a series of 4-(indol-5-ylamino)thieno[2,3-b]pyridine-5-carbonitriles. Bioorg. Med. Chem. Lett. 2008, 18, 2850–2853. [Google Scholar]
- Nathan, T.L.; Boschelli, D.H.; Lee, J.; Chaudhary, D. 2-Alkenylthieno[2,3-b]pyridine-5-carbonitriles: Potent and selective inhibitors of PKCtheta. Bioorg. Med. Chem. Lett. 2008, 18, 4420–4423. [Google Scholar]
- Madhusudana, K.; Shireesha, B.; Naidu, V.G.; Ramakrishna, S.; Narsaiah, B.; Rao, A.R.; Diwan, P.V. Anti-inflammatory potential of thienopyridines as possible alternative to NSAIDs. Eur. J. Pharmacol 2012, 678, 48–54. [Google Scholar]
- Liu, H.; Li, Y.; Wang, X.Y.; Wang, B.; He, H.Y.; Liu, J.Y.; Xiang, M.L.; He, J.; Wu, X.H.; Yang, L. Synthesis, preliminary structure-activity relationships, and in vitro biological evaluation of 6-aryl-3-amino-thieno[2,3-b]pyridine derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2013, 23, 2349–2352. [Google Scholar]
- Bernardino, A.M.; da Silva Pinheiro, L.C.; Rodrigues, C.R.; Loureiro, N.I.; Castro, H.C.; Lanfredi-Rangel, A.; Sabatini-Lopes, J.; Borges, J.C.; Carvalho, J.M.; Romeiro, G.A.; et al. Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino)thieno[2,3-b]pyridine derivatives. Bioorg. Med. Chem. 2006, 14, 5765–5770. [Google Scholar]
- Al-Trawneh, S.A.; El-Abadelah, M.M.; Zahra, J.A.; Al-Taweel, S.A.; Zani, F.; Incerti, M.; Cavazzoni, A.; Vicini, P. Synthesis and biological evaluation of tetracyclic thienopyridones as antibacterial and antitumor agents. Bioorg. Med. Chem. 2011, 19, 2541–2548. [Google Scholar]
- Moretto, A.F.; Kirincich, S.J.; Xu, W.X.; Smith, M.J.; Wan, Z.K.; Wilson, D.P.; Follows, B.C.; Binnun, E.; Joseph-McCarthy, D.; Foreman, K.; et al. Bicyclic and tricyclic thiophenes as protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. 2006, 14, 2162–2177. [Google Scholar]
- Bahekar, R.H.; Jain, M.R.; Goel, A.; Patel, D.N.; Prajapati, V.M.; Gupta, A.A.; Jadav, P.A.; Patel, P.R. Design, synthesis, and biological evaluation of substituted-N-(thieno[2,3-b]pyridin-3-yl)-guanidines, N-(1H-pyrrolo[2,3-b]pyridin-3-yl)-guanidines, and N-(1H-indol-3-yl)-guanidines. Bioorg. Med. Chem. 2007, 15, 3248–3265. [Google Scholar]
- Bahekar, R.H.; Jain, M.R.; Jadav, P.A.; Prajapati, V.M.; Patel, D.N.; Gupta, A.A.; Sharma, A.; Tom, R.; Bandyopadhya, D.; Modi, H.; et al. Synthesis and antidiabetic activity of 2,5-disubstituted-3-imidazol-2-yl-pyrrolo[2,3-b]pyridines and thieno[2,3-b]pyridines. Bioorg. Med. Chem. 2007, 15, 6782–6795. [Google Scholar]
- Kamata, M.; Yamashita, T.; Kina, A.; Funata, M.; Mizukami, A.; Sasaki, M.; Tani, A.; Funami, M.; Amano, N.; Fukatsu, K. Design, synthesis, and structure-activity relationships of novel spiro-piperidines as acetyl-CoA carboxylase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3643–3647. [Google Scholar]
- Adachi, I.; Hiramatsu, Y.; Ueda, M.; Kawakami, M. 4,7-Dihydrothieno[2,3-b] Pyridine Derivatives Useful in the Treatment of Cardiovascular Diseases. U.S. 4703-051 A. 1986. [Google Scholar]
- Adachi, I.; Yamamori, T.; Hiramatsu, Y.; Sakai, K.; Mihara, S.; Kawakami, M.; Masui, M.; Uno, O.; Ueda, M. Studies on dihydropyridines. III. Synthesis of 4,7-dihydrothieno [2,3-b]-pyridines with vasodilator and antihypertensive activities. Chem. Pharm. Bull 1988, 36, 4389–4402. [Google Scholar]
- Ueda, M.; Matsumura, S.; Masui, M.; Matsuura, E.; Kawakami, M.; Fujitomo, H.; Umeda, T.; Kagawa, H; Hirohata, S.; Shima, K.; et al. Pharmacological studies on a new dihydrothienopyridine calcium antagonist. 3rd communication: antihypertensive effects of S-(+)-methyl-4,7-dihydro-3-isobutyl-6-methyl-4-(3-nitrophenyl)thieno[2,3-b] pyridine-5-carboxylate in hypertensive rats and dogs. Arzneimittelforschung 1993, 43, 1282–1290. [Google Scholar]
- Ohba, S.; Nakajima, K.; Komiyama, Y.; Kugimiya, F.; Igawa, K.; Itaka, K.; Moro, T.; Nakamura, K.; Kawaguchi, H.; Takato, T.; et al. A novel osteogenic helioxanthin-derivative acts in a BMP-dependent manner. Biochem. Biophys. Res. Commun. 2007, 357, 854–860. [Google Scholar]
- Saito, K.; Nakao, A.; Shinozuka, T.; Shimada, K.; Matsui, S.; Oizumi, K.; Yano, K.; Ohata, K.; Nakai, D.; Nagai, Y.; et al. Discovery and structure-activity relationship of thienopyridine derivatives as bone anabolic agents. Bioorg. Med. Chem. 2013, 21, 1628–1642. [Google Scholar]
- Buchstaller, H.P.; Siebert, C.D.; Steinmetz, R.; Frank, I.; Berger, M.L.; Gottschlich, R.; Leibrock, J.; Krug, M.; Steinhilber, D.; Noe, C.R. Synthesis of thieno[2,3-b]pyridinones acting as cytoprotectants and as inhibitors of [3H]glycine binding to the N-methyl-D-aspartate (NMDA) receptor. J. Med. Chem. 2006, 49, 864–871. [Google Scholar]
- Mohler, E.G.; Shacham, S.; Noiman, S.; Lezoualc’h, F.; Robert, S.; Gastineau, M.; Rutkowski, J.; Marantz, Y.; Dumuis, A.; Bockaert, J.; et al. VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 2007, 53, 563–573. [Google Scholar]
- Le, U.; Melancon, B.J.; Bridges, T.M.; Vinson, P.N.; Utley, T.J.; Lamsal, A.; Rodriguez, A.L.; Venable, D.; Sheffler, D.J.; Jones, C.K.; et al. Discovery of a selective M(4) positive allosteric modulator based on the 3-amino-thieno[2,3-b]pyridine-2-carboxamide scaffold: Development of ML253, a potent and brain penetrant compound that is active in a preclinical model of schizophrenia. Bioorg. Med. Chem. Lett. 2013, 23, 346–350. [Google Scholar]
- Patrick, G.L.; Kinsmar, O.S. Synthesis and antifungal activity of novel azo-d-homosteroids, hydroisoquinolines, pyridines and hydropyridines. Eur. J. Med. Chem. Chim. Ther. 1996, 31, 615–624. [Google Scholar]
- Hishmat, O.H.; Abdel Galil, F.M.; Farrag, D.S. Synthesis and antimicrobial activity of new benzofuranylpyridine derivatives. Pharmazie 1990, 45, 793–795. [Google Scholar]
- Doshi, R.; Kagthara, P.; Parekh, H. Synthesis and biological evaluation of some novel isooxazoles and cyanopyridine a new class of potential anti-tubercular agents. Indian J. Chem. 1999, 38, 348–352. [Google Scholar]
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a]pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar]
- Datta, N.J.; Khunt, R.C.; Parikh, A.R. Aryl amides: Preparation and antimicrobial evaluation. Inst. Chem. India 2000, 72, 133–134. [Google Scholar]
- Nagashree, S.; Mallesha, L.; Mallu, P. Synthesis and in vitro biological activity of 6-chloropyridin-2-ylamine derivatives. Der Pharma Chemica 2013, 5, 50–55. [Google Scholar]
- Abdelhamid, A.O.; Fahmi, A.A.; Halim, K.N.M. Design and synthesis of Some New Pyrazolo[1,5-a]pyrimidines, Pyrazolo[5,1-c]triazines, Pyrazolo-[3,4-d]pyridazines, Oxazolo[3,4-d]pyridazines containing pyrazole moiety. Synth. Commun. 2013, 43, 1101–1126. [Google Scholar]
- Abdel-Aziem, A.; El-Gendy, M.S.; Abdelhamid, A.O. Synthesis and antimicrobial activities of pyrido[2,3-d]pyrimidine, triazolopyridopyrimidine, triazolopyrimidine, and pyrido[2,3-d:6,5d']dipyrimidine derivatives. Eur. J. Chem. 2012, 3, 455–460. [Google Scholar]
- Gomha, S.M.; Shawali, A.S.; Abdelhamid, A.O. Convenient Methods for synthesis of various fused heterocycles via utility of 4-Acetyl-5-methyl-1-phenyl-pyrazole as precursor. Turk. J. Chem. 2014, 38, 865–879. [Google Scholar]
- Ahmad, S.A.; Abdelriheem, N.A.; Abdelhamid, A.O. Cycloketones as precursor for convenient synthesis of azolopyrimidines and 5-arylazothiazoles. Eur. Bull Chem. 2014, 3, 1038–1045. [Google Scholar]
- Ahmed, A.S.; Ahmed, O.M.; Abdelhamid, A.O. Synthesis and anti-tumor activities of some new [1,2,4]triazolo[1,5-a]pyrimidines. Eur. J. Chem. 2014, 5, 334–338. [Google Scholar]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; El-Idreesy, T.T.; Rashdan, H.R.M. Green One Pot Solvent-Free Synthesis of Pyrazolo[1,5-a]pyrimidines, Azolo[3,4-d]pyridiazines and thieno[2,3-b]pyridines containing triazole moiety. Int. J. Adv. Res. 2013, 1, 729–745. [Google Scholar]
- Abdelhamid, A.O.; Fahmi, A.A.; Baaiu, B.S. Synthesis of some new pyrazolo[3,4-d]pyridiazines, Oxazolo[3,4-d]pyridazines, Azolo[1,5-a]pyrimidines, Azolo[5,1-c]triazines, Pyrazoles and Benzo[b][l,4]diazepine Incorporated 5-Bromobenzofuran-2-yl Moiety. J. Heterocycl. Chem 2015, in press. [Google Scholar]
- Studennikova, L.D. Hydrazones of acetaceric ester. Sb. Nauch Ref. Zh. Kim. 1969, 1, 71–73. [Google Scholar]
- Sharma, R.N.; Sharma, K.P.; Dixit, S.N. Synthesis, Characterization, and Biological activities of Some New Arylazopyrazoles. Int. J. ChemTech Res. 2010, 2, 800–806. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–c, 6a–d, 7–9, 10–18, 20–23 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelriheem, N.A.; Ahmad, S.A.-K.; Abdelhamid, A.O. Synthesis of some new Thieno[2,3-b]pyridines, Pyrimidino[4',5':4,5]thieno[2,3-b]pyridine and Pyridines Incorporating 5-Bromobenzofuran-2-yl Moiety. Molecules 2015, 20, 822-838. https://doi.org/10.3390/molecules20010822
Abdelriheem NA, Ahmad SA-K, Abdelhamid AO. Synthesis of some new Thieno[2,3-b]pyridines, Pyrimidino[4',5':4,5]thieno[2,3-b]pyridine and Pyridines Incorporating 5-Bromobenzofuran-2-yl Moiety. Molecules. 2015; 20(1):822-838. https://doi.org/10.3390/molecules20010822
Chicago/Turabian StyleAbdelriheem, Nadia Abdelhamed, Sayed Abdel-Kader Ahmad, and Abdou Osman Abdelhamid. 2015. "Synthesis of some new Thieno[2,3-b]pyridines, Pyrimidino[4',5':4,5]thieno[2,3-b]pyridine and Pyridines Incorporating 5-Bromobenzofuran-2-yl Moiety" Molecules 20, no. 1: 822-838. https://doi.org/10.3390/molecules20010822