Pinelliae Rhizoma, a Toxic Chinese Herb, Can Significantly Inhibit CYP3A Activity in Rats
Abstract
:1. Introduction
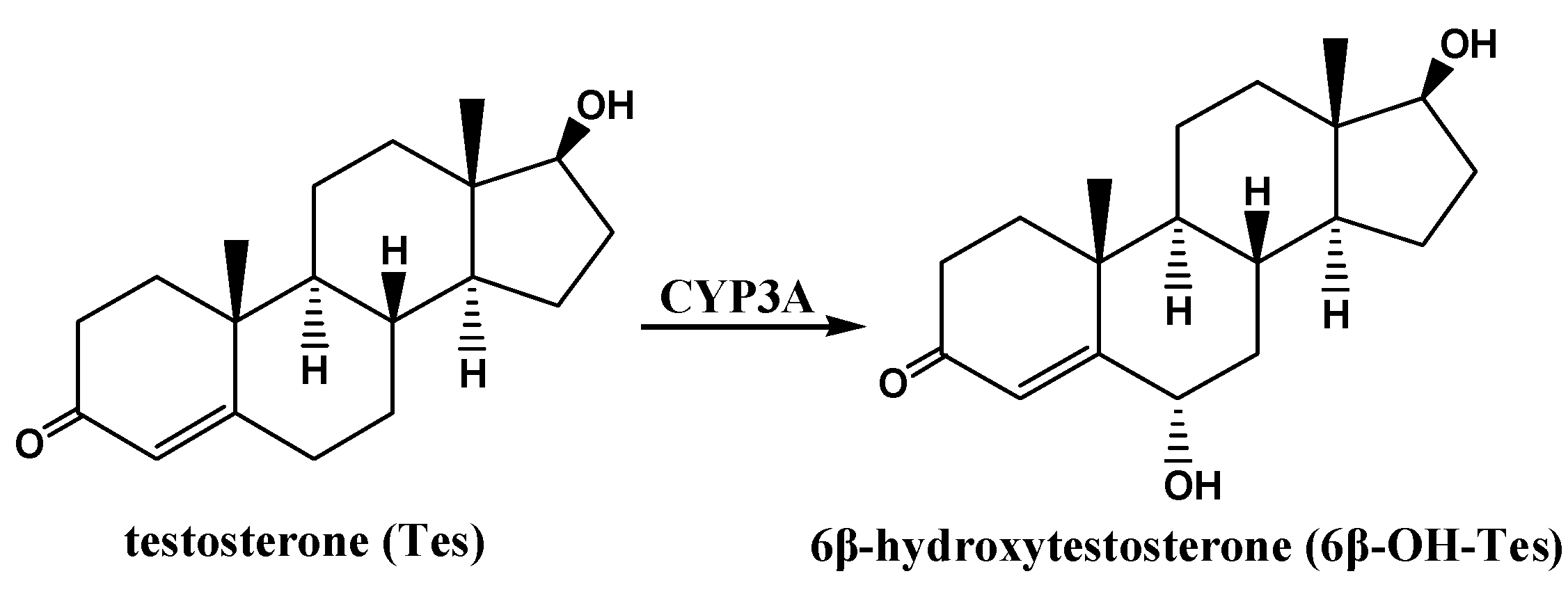
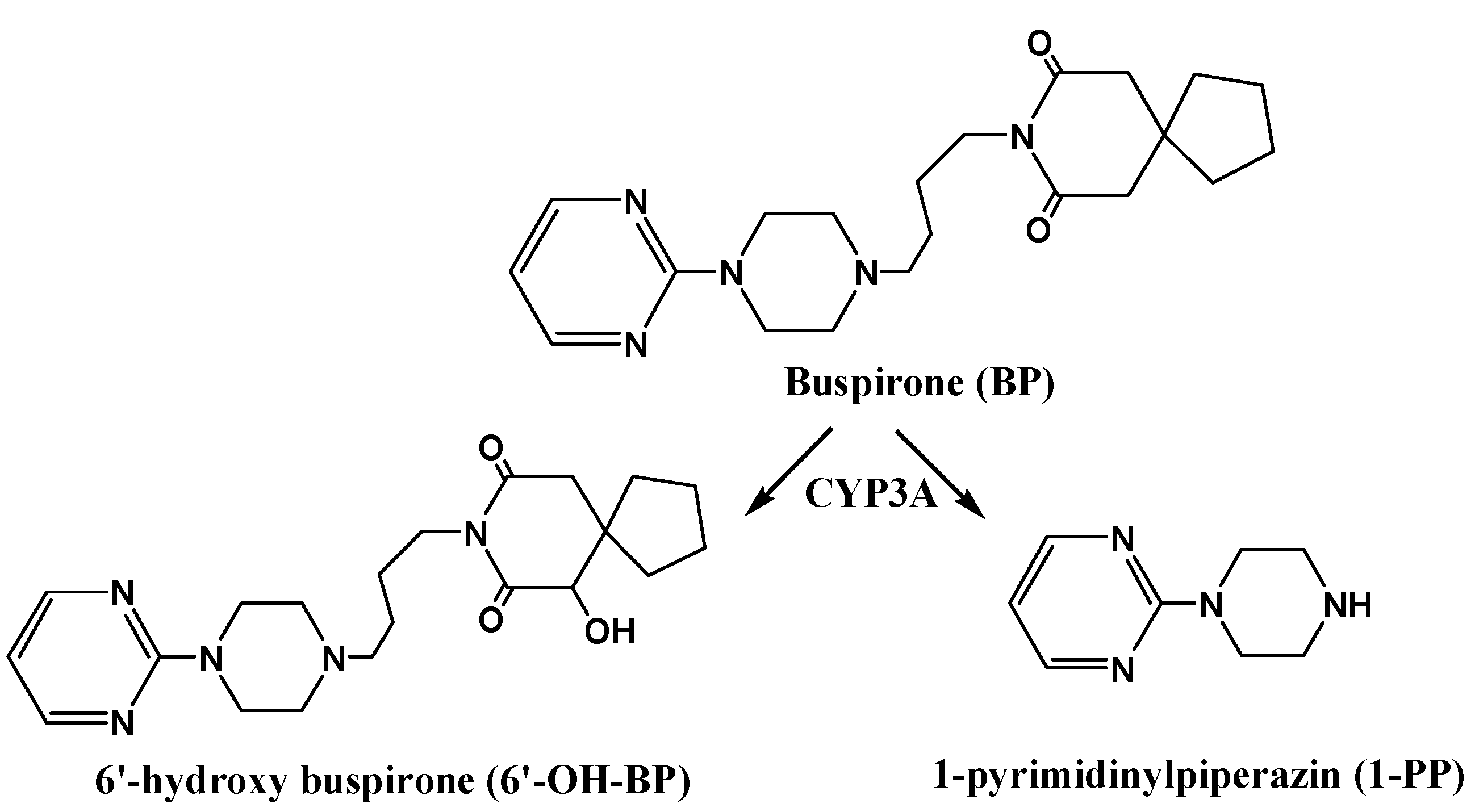
2. Results and Discussion
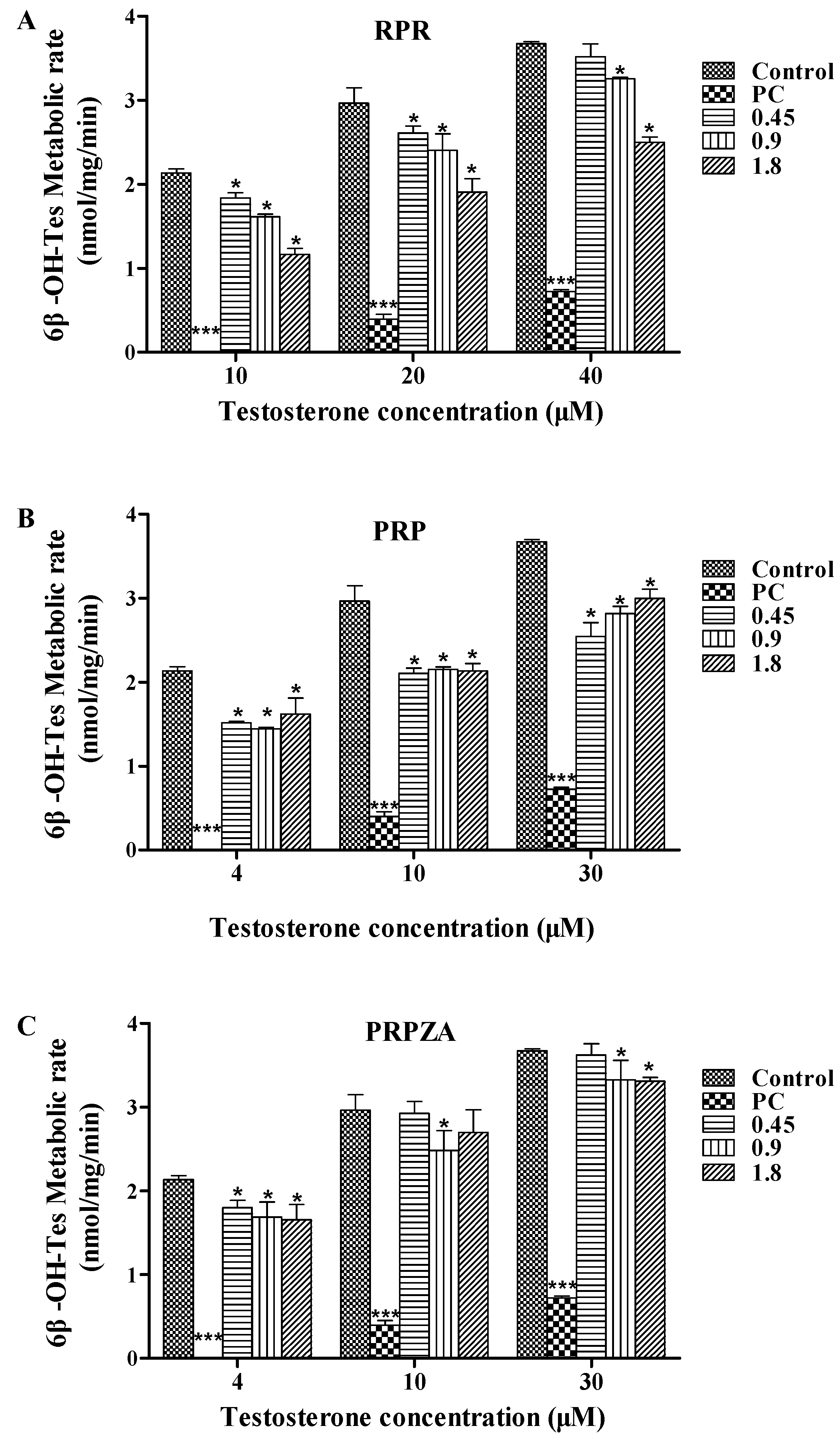
2.1. Effects of RPR, PRP or PRPZA on Hepatic CYP3A Activity ex Vivo
2.1.1. Effects of RPR on Hepatic CYP3A Activity ex Vivo
2.1.2. Effects of PRP on Hepatic CYP3A Activity ex Vivo
2.1.3. Effects of PRPZA on Hepatic CYP3A Activity ex Vivo
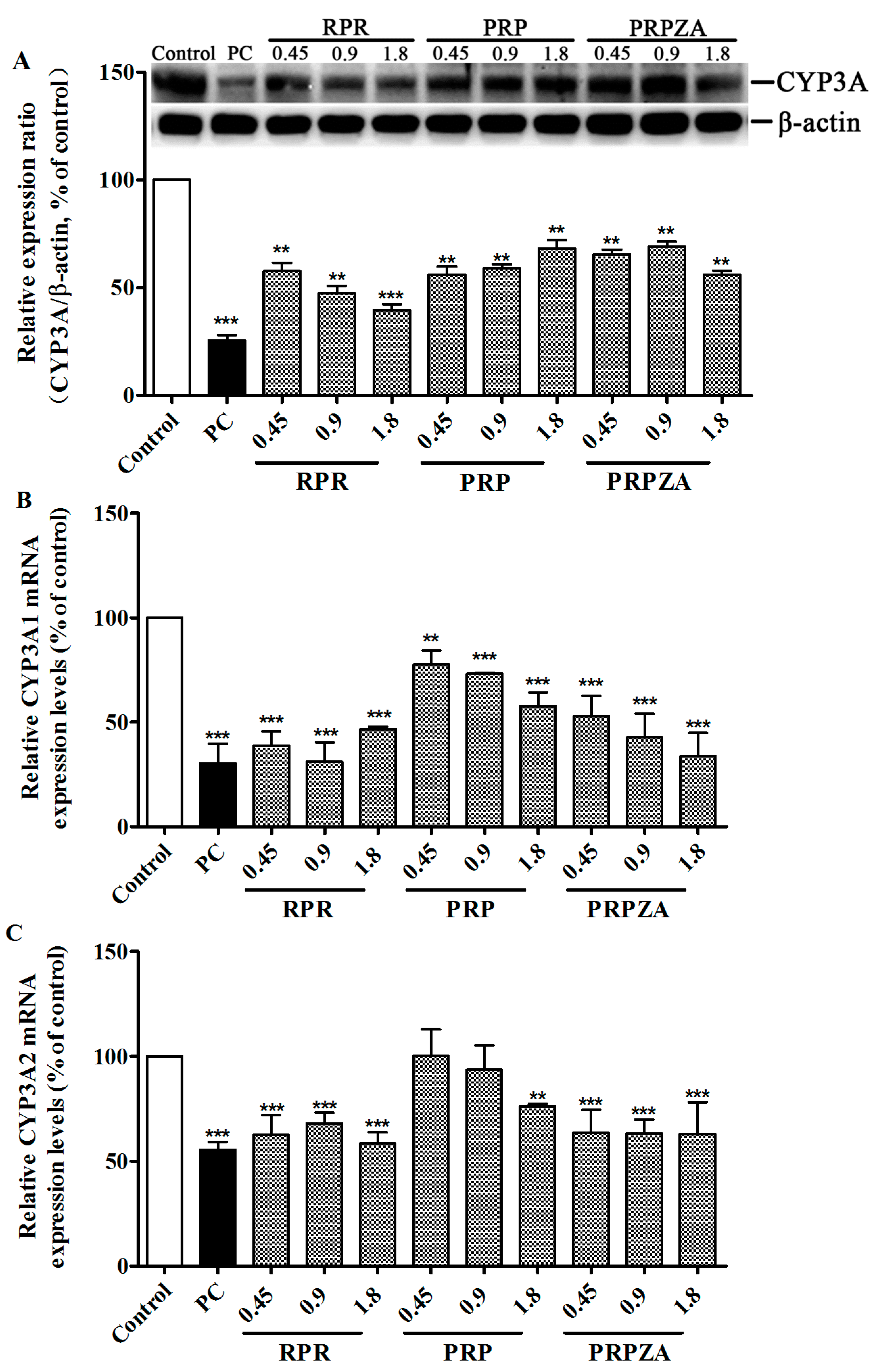
2.2. Effects of RPR, PRP or PRPZA on Hepatic CYP3A Protein and mRNA Expression Levels
2.2.1. Effects of RPR, PRP or PRPZA on Hepatic CYP3A Protein Expression Levels
2.2.2. Effects of RPR, PRP or PRPZA on Hepatic CYP3A1 mRNA Expression Levels
2.2.3. Effects of RPR, PRP or PRPZA on Hepatic CYP3A2 mRNA Expression Levels
2.3. Effects of RPR, PRP or PRPZA on BP Pharmacokinetics in Vivo

| Variable | Control | RPR | PRP | PRPZA |
|---|---|---|---|---|
| BP | ||||
| AUC0-t (μg·min·mL−1) | 14,774.65 ± 2767.74 | 16,253.05 ± 4045.20 | 16,146.91 ± 953.18 | 17,502.24 ± 4206.67 |
| Cmax (ng·mL−1) | 681.16 ± 253.80 | 672.76 ± 113.10 | 702.01 ± 181.79 | 783.03 ± 121.97 |
| t1/2 (min) | 118.15 ± 9.83 | 150.54 ± 34.79 | 146.12 ± 15.33 | 96.79 ± 8.02 |
| CL (mL·min−1·kg−1) | 34.37 ± 6.05 | 32.01 ± 7.99 | 30.53 ± 1.90 | 29.74 ± 7.03 |
| Vd (L·kg−1) | 5806.61 ± 752.26 | 7263.06 ± 3444.26 | 6436.20 ± 729.96 | 4175.27 ± 1125.25 |
| 6'-OH-BP | ||||
| AUC0-t (μg·min·mL−1) | 3515.02 ± 1032.97 | 3741.64 ± 811.23 | 4405.57 ± 1757.76 | 4468.04 ± 1787.44 |
| Cmax (ng·mL−1) | 38.96 ± 17.86 | 40.05 ± 16.09 | 43.66 ± 22.07 | 41.43 ± 18.16 |
| t1/2 (min) | 43.62 ± 11.61 | 62.62 ± 21.72 | 38.23 ± 9.75 | 40.78 ± 10.74 |
| tmax (min) | 42.00 ± 26.83 | 27.00 ± 6.71 | 51.00 ± 36.12 | 60.00 ± 21.21 |
| CL (mL·min−1·kg−1) | 151.43 ± 39.84 | 139.88 ± 36.57 | 131.59 ± 58.71 | 126.79 ± 49.62 |
| Vd (L·kg−1) | 9667.04 ± 4169.74 | 13,323.56 ± 7985.70 | 7388.06 ± 3679.70 | 7013.16 ± 1855.36 |
| 6'-OH-BP/BP ratio | 0.24 ± 0.06 | 0.24 ± 0.08 | 0.28 ± 0.11 | 0.25 ± 0.05 |
| 1-PP | ||||
| AUC0-t (μg·min·mL−1) | 4249.31 ± 1290.50 | 3624.86 ± 1142.96 | 4958.20 ± 1259.90 | 2596.72 ± 557.34 |
| Cmax (ng·mL−1) | 27.35 ± 12.57 | 17.35 ± 7.20 | 25.99 ± 4.86 | 13.93 ± 3.87 |
| t1/2 (min) | 160.16 ± 52.85 | 244.73 ± 76.46 | 280.52 ± 308.26 | 268.82 ± 55.17 |
| tmax (min) | 48.00 ± 26.83 | 51.00 ± 29.24 | 78.00 ± 16.43 | 54.00 ± 25.10 |
| CL (mL·min−1·kg−1) | 126.20 ± 36.57 | 143.18 ± 56.52 | 97.07 ± 39.69 | 198.43 ± 34.45 |
| Vd (L·kg−1) | 30,074.20 ± 16,188.54 | 46,942.34 ± 11,037.16 | 25,588.78 ± 11,512.25 | 69,192.39 ± 18,332.26 |
| 1-PP/BP ratio | 0.30 ± 0.11 | 0.22 ± 0.04 | 0.31 ± 0.08 | 0.15 ± 0.03 ** |
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Animals
3.4. Preparation of Herbs
3.5. Preparation of Rat Liver Microsomes (RLMs)
3.6. Determination of CYP3A Activity in RLMs by Tes Metabolism ex Vivo
3.7. CYP3A Protein Expression of RLMs by Western Blot
3.8. CYP3A1/2 mRNA Measurement by Real-Time PCR
3.9. Pharmacokinetics of BP in Rats with RPR, PRP or PRPZA Water Extracts Pretreatment for 7 days
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ok, I.S.; Kim, S.H.; Kim, B.K.; Lee, J.C.; Lee, Y.C. Pinellia ternata, Citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediat. Inflamm. 2009, 2009. [Google Scholar] [CrossRef]
- Kurata, K.; Tai, T.; Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Watanabe, K.; Nunoura, Y. Quantitative analysis of anti-emetic principle in the tubers of Pinellia ternata by enzyme immunoassay. Planta Med. 1998, 64, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.D.; Liu, N.; Guo, P.; Guo, L.Y.; Sun, Y.; Shi, J.; Wang, L. Analysis on clinical treatment in hypertension by traditional Chinese medicine for 10 years in Beijing. Zhongguo Zhong Yao Za Zhi 2007, 32, 1569–1572. [Google Scholar] [PubMed]
- Wu, X.Y.; Zhao, J.L.; Zhang, M.; Li, F.; Zhao, T.; Yang, L.Q. Sedative, hypnotic and anticonvulsant activities of the ethanol fraction from Rhizoma Pinelliae Praeparatum. J. Ethnopharmacol. 2011, 135, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.S.; Zhu, R.R. Brief discussion on modern pharmaceutical research progress and compatibility changes of Radix Aconiti Lateralis Preparata and Rhizoma Pinelliae. China Pharm. 2012, 21, 19–20. [Google Scholar]
- Zhou, S.S.; Ma, Z.C.; Liang, Q.D.; Wang, Y.G.; Tan, H.L.; Xiao, C.R.; Zhang, B.L.; Gao, Y. UPLC/Q-TOF-MS based chemical profiling approach to evaluate chemical composition of augmentation toxicity in combination of Radix Aconitiand Pinellia Praeparata. Acta Chim. Sin. 2012, 70, 284–290. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; Han, H.; Ji, R.; Ye, D.J. Studies on stimulating components of raw Pinellia ternata (Thunb.) (banxia). Zhongguo Zhong Yao Za Zhi 1999, 24, 763. [Google Scholar]
- Zhong, L.Y.; Wu, H.; Zhang, K.W.; Wang, Q.R. Study on irritation of calcium oxalate crystal in raw Pinellia ternata. Zhongguo Zhong Yao Za Zhi 2006, 31, 1706–1710. [Google Scholar] [PubMed]
- Zhong, L.Y.; Wu, H. Current researching situation of mucosal irritant compontents in Araceae family plants. Zhongguo Zhong Yao Za Zhi 2006, 31, 1561–1563. [Google Scholar] [PubMed]
- Ge, X.Y.; Wu, H. phytochemical properties and quality evaluation methods of Pinelliae ternate. China Pharm. 2009, 18, 3–5. [Google Scholar]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Ohara, N.; Takagi, H.; Hoshino, S. Characterization of an acidic polysaccharide with immunological activities from the tuber of Pinellia ternata. Biol. Pharm. Bull. 1994, 17, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, M.; Gonda, R.; Ohara, N.; Shimizu, N.; Shishido, C.; Fujiki, Y. A glucan having reticuloendothelial system-potentiating and anti-complementary activities from the tuber of Pinellia ternata. Biol. Pharm. Bull. 1994, 17, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Li, M.; Shi, J.; Xia, D.M.; Li, X.X.; Yang, X.Y. A system research on HPLC characteristic fingerprint of Pinelliae Rhizoma and Pinelliae Rhizoma Praeparatum cum Zingibere et Alumine. Chin. Tradit. Herb. Drugs 2014, 45, 652–658. [Google Scholar]
- Cheng, Z.X.; Wu, J.J.; Liu, Z.Q.; Lin, N. Development of a hydrophilic interaction chromatography-UPLC assay to determine trigonelline in rat plasma and its application in a pharmacokinetic study. Chin. J. Nat. Med. 2013, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.F.; Hong, X.K.; Wang, Z.H. Progress of studies on preparation of Rhizoma Pinelliae. Chin. Tradit. Patent Med. 2004, 26, 38–40. [Google Scholar]
- Cai, B.C.; Qin, K.M.; Wu, H.; Cai, H.; Lu, T.L.; Zhang, X.D. Chemical mechanism during Chinese medicine processing. Prog. Chem. 2012, 24, 637–649. [Google Scholar]
- Pei, M.; Duan, X.; Pei, X. Compatability chemistry of acid-alkaline pair medicine of Fuzi and Gancao in Sini decoction. Zhongguo Zhong Yao Za Zhi 2009, 34, 2047–2050. [Google Scholar] [PubMed]
- Zhang, J.M.; Liao, W.; He, Y.X.; He, Y.; Yan, D.; Fu, C.M. Study on intestinal absorption and pharmacokinetic characterization of diester diterpenoid alkaloids in precipitation derived from fuzi-gancao herb-pair decoction for its potential interaction mechanism investigation. J. Ethnopharmacol. 2013, 147, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Soderberg Lofdal, K.C.; Andersson, M.L.; Gustafsson, L.L. Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs 2013, 73, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Atayee, R.S.; Best, B.M.; Ma, J.D. Observations on the urine metabolic profile of codeine in pain patients. J. Anal. Toxicol. 2014, 38, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cheng, Z.Y.; He, J.; Jia, L.J.; Qiao, H.L. Concentration-dependent inhibitory effects of baicalin on the metabolism of dextromethorphan, a dual probe of CYP2D and CYP3A, in rats. Chem. Biol. Interact. 2013, 203, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Fancher, R.M.; Zhang, H.; Gan, J. Mechanism-based inhibition of human cytochrome P4503A4 by domperidone. Xenobiotica 2010, 40, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wojcikowski, J.; Boksa, J.; Daniel, W.A. Main contribution of the cytochrome P450 isoenzyme 1A2 (CYP1A2) to N-demethylation and 5-sulfoxidation of the phenothiazine neuroleptic chlorpromazine in human liver—A comparison with other phenothiazines. Biochem. Pharmacol. 2010, 80, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, N.; Senda, C.; Kishimoto, W.; Sakai, K.; Funae, Y.; Igarashi, T. Identification of CYP3A4 as the predominant isoform responsible for the metabolism of ambroxol in human liver microsomes. Xenobiotica 2000, 30, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Ernst, E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009, 69, 1777–1798. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Tripathi, K.P.; Roy, S.; Khan, F.; Sharma, A. Pharmacovigilance: Effects of herbal components on human drugs interactions involving cytochrome P450. Bioinformation 2008, 3, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Kim, S.A.; Kim, I.S.; Ko, S.G. The Evaluation of CP-001 (a standardized herbal mixture of Houttuynia cordata, Rehmannia glutinosa, Betula platyphylla, and Rubus coreanus) for cytochrome P450-related herb-drug interactions. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Chen, X.W.; Sneed, K.B.; Pan, S.Y.; Cao, C.; Kanwar, J.R.; Chew, H.; Zhou, S.F. Herb-drug interactions and mechanistic and clinical considerations. Curr. Drug Metab. 2012, 13, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Fujita, K.; Murayama, N.; Akiyama, Y.; Yamazaki, H.; Sasaki, Y. Sorafenib and sunitinib, two anticancer drugs, inhibit CYP3A4-mediated and activate CY3A5-mediated midazolam 1'-hydroxylation. Drug Metab. Dispos. 2011, 39, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: The past, present and future. Trends Pharmacol. Sci. 2004, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Shou, M.; Lu, T.; Krausz, K.W.; Sai, Y.; Yang, T.; Korzekwa, K.R.; Gonzalez, F.J.; Gelboin, H.V. Use of inhibitory monoclonal antibodies to assess the contribution of cytochromes P450 to human drug metabolism. Eur. J. Pharmacol. 2000, 394, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, G.; Rose, T.; Rissler, K. Determination of testosterone metabolites in human hepatocytes. I. Development of an on-line sample preparation liquid chromatography technique and mass spectroscopic detection of 6beta-hydroxytestosterone. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 784, 49–61. [Google Scholar] [CrossRef]
- Chen, X.M.; Wei, M.; Zhang, H.M.; Luo, C.H.; Chen, Y.K.; Chen, Y. Effect of vanillin and ethyl vanillin on cytochrome P450 activity in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, X.; Zhou, J.; Tang, L.; Xia, B.; Hu, M.; Zhou, F.; Liu, Z. The exposure of highly toxic aconitine does not significantly impact the activity and expression of cytochrome P450 3A in rats determined by a novel ultra performance liquid chromatography-tandem mass spectrometric method of a specific probe buspirone. Food Chem. Toxicol. 2013, 51, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Debri, K.; Boobis, A.R.; Davies, D.S.; Edwards, R.J. Distribution and induction of CYP3A1 and CYP3A2 in rat liver and extrahepatic tissues. Biochem. Pharmacol. 1995, 50, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, Z.; Zhu, L.; Lu, L.; Zhang, G.; Wang, Y.; Xu, Y.; Lin, N.; Liu, Z. Coadministration of Pinellia ternata can significantly reduce Aconitum carmichaelii to inhibit CYP3A activity in Rats. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Chen, J.; Halls, S.C.; Alfaro, J.F.; Zhou, Z.; Hu, M. Potential beneficial metabolic interactions between tamoxifen and isoflavones via cytochrome P450-mediated pathways in female rat liver microsomes. Pharm. Res. 2004, 21, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Cheng, Z.X.; Liu, Z.Q.; Lin, N. Influence of Wutou Tang on Activity of CYP3A4 in Rat Liver Microsomes. Chin. J. Exp. Tradit. Med. Formul. 2013, 19, 233–236. [Google Scholar]
- Sample Availability: Samples of the RPR, PRP and PRPZA herbs are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Cheng, Z.; He, S.; Shi, J.; Liu, S.; Zhang, G.; Zhu, L.; Liu, L.; Liu, Z.; Lin, N.; et al. Pinelliae Rhizoma, a Toxic Chinese Herb, Can Significantly Inhibit CYP3A Activity in Rats. Molecules 2015, 20, 792-806. https://doi.org/10.3390/molecules20010792
Wu J, Cheng Z, He S, Shi J, Liu S, Zhang G, Zhu L, Liu L, Liu Z, Lin N, et al. Pinelliae Rhizoma, a Toxic Chinese Herb, Can Significantly Inhibit CYP3A Activity in Rats. Molecules. 2015; 20(1):792-806. https://doi.org/10.3390/molecules20010792
Chicago/Turabian StyleWu, Jinjun, Zaixing Cheng, Shugui He, Jian Shi, Shuqiang Liu, Guiyu Zhang, Lijun Zhu, Liang Liu, Zhongqiu Liu, Na Lin, and et al. 2015. "Pinelliae Rhizoma, a Toxic Chinese Herb, Can Significantly Inhibit CYP3A Activity in Rats" Molecules 20, no. 1: 792-806. https://doi.org/10.3390/molecules20010792





