Deoxypodophyllotoxin Induces G2/M Cell Cycle Arrest and Apoptosis in SGC-7901 Cells and Inhibits Tumor Growth in Vivo
Abstract
:1. Introduction
2. Results
2.1. DPT Inhibited the Proliferation of SGC-7901 Cells

2.2. DPT Destabilized Microtubules Assembly

2.3. DPT Induced Apoptosis in SGC-7901 Cells
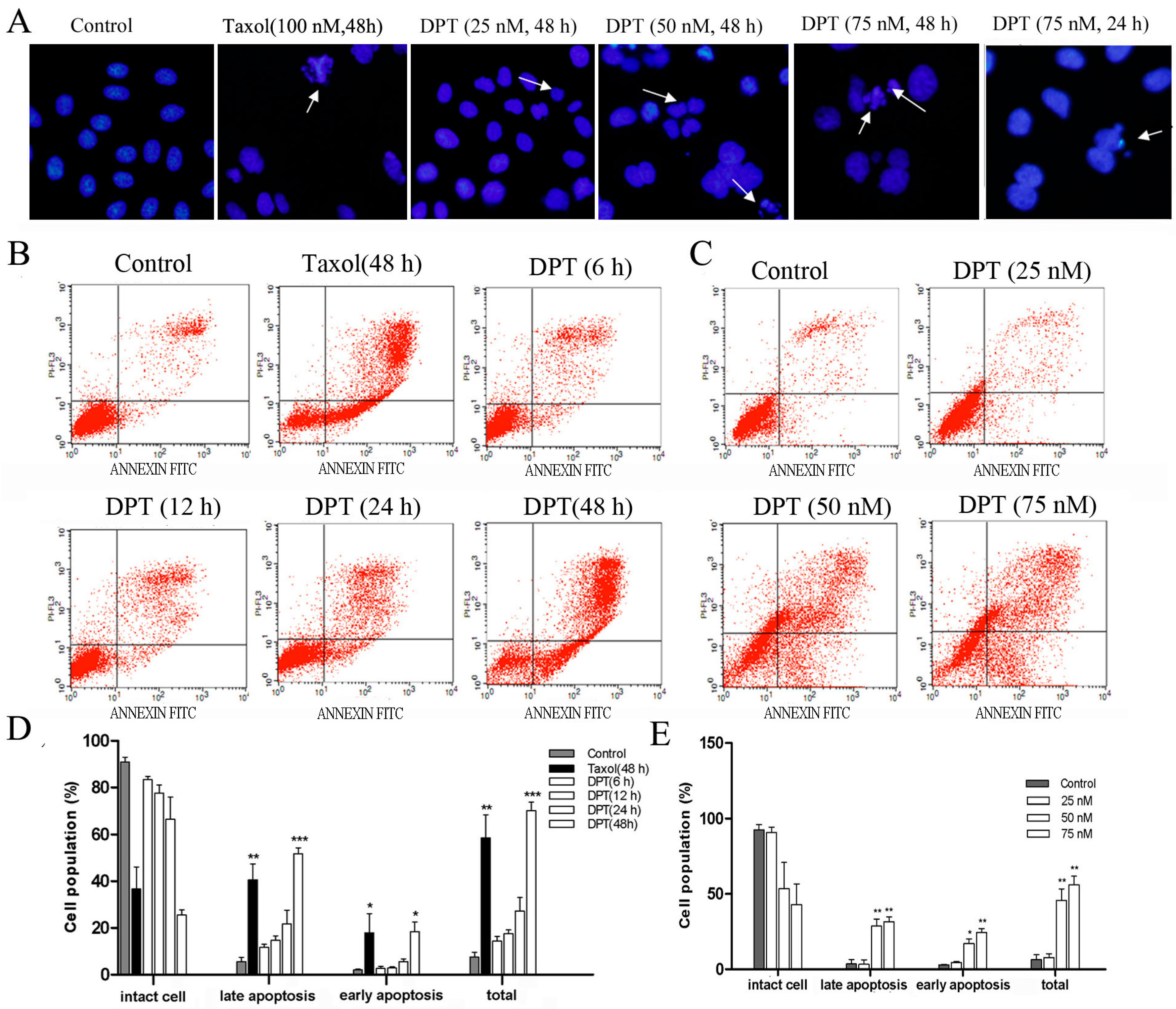
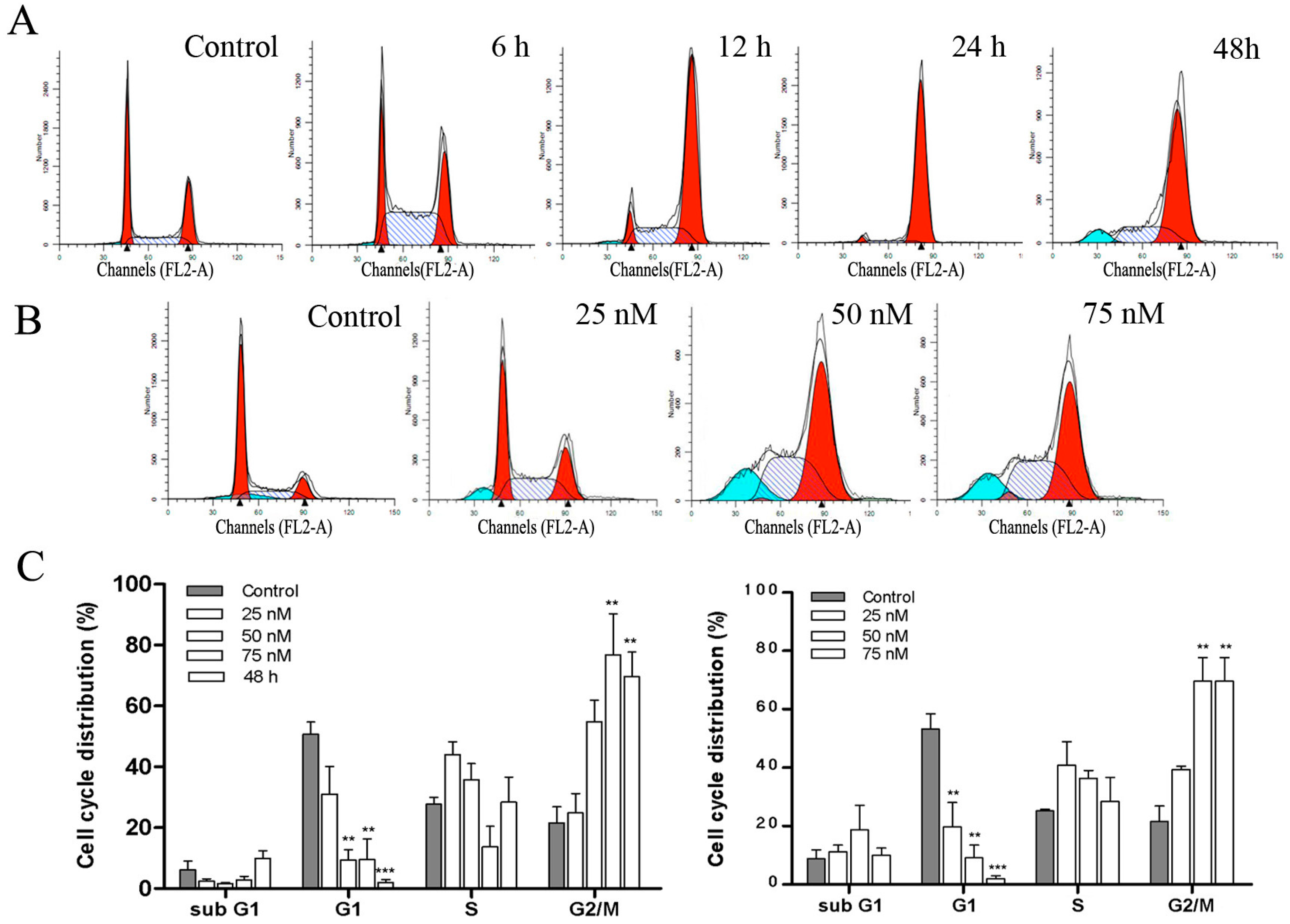
2.4. DPT Induced G2/M Cell Cycle Arrest in SGC-7901 Cells
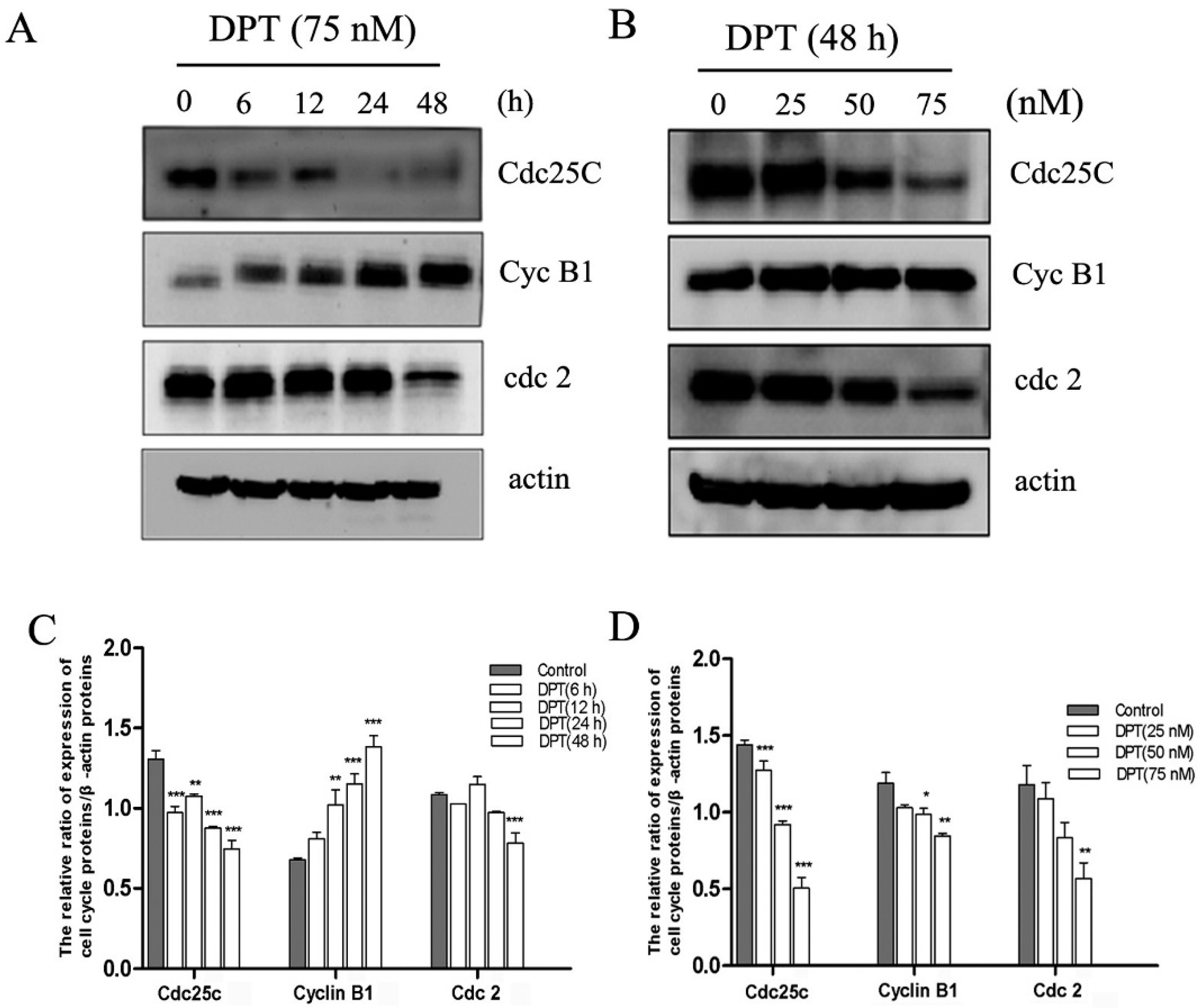
2.5. Effects of DPT on the Expression of Cell Cycle Regulatory Proteins
2.6. DPT Induced Caspase Activation and Inactivation of Bcl-2 Protein

2.7. DPT Possessed Potent Anticancer Activity in Vivo

| Group | Dosage (mg/kg) | Tumor Weight (g) | Inhibition Rate (%) |
|---|---|---|---|
| Docetaxel | 20 | 0.83 ± 0.12 * | 42.63 |
| Etoposide | 20 | 0.68 ± 0.16 ** | 53.11 |
| DPT | 5 | 1.12 ± 0.03 * | 22.19 |
| 10 | 0.75 ± 0.06 * | 47.91 | |
| 20 | 0.71 ± 0.11 * | 50.93 |
2.8. DPT Decreased Microvessel Density (MVD)
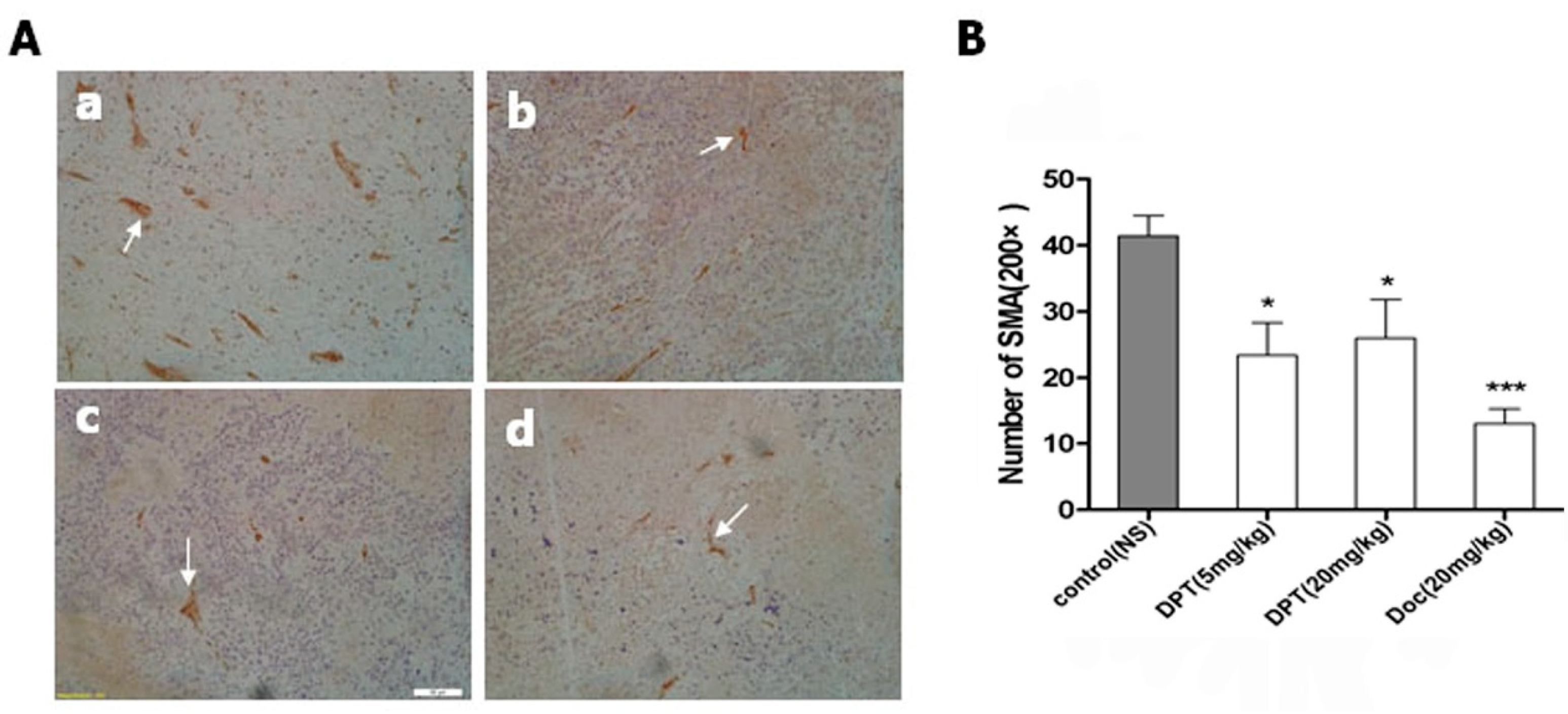
3. Discussion
4. Experimental
4.1. Materials
4.2. Cell Culture
4.3. Animals
4.4. Immunofluorescence Analysis
4.5. Cell Proliferation Analysis
4.6. Cell Cycle Analysis
4.7. Apoptosis Detection
4.8. Western Blot Analysis
4.9. Caspase-3 Activity Assay
4.10. In Vivo Study
4.11. Quantification of Microvessel Density
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The national cancer data base report on poor survival of U.S. Gastric carcinoma patients treated with gastrectomy: Fifth edition american joint committee on cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000, 88, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Stenner-Liewen, F.; Zippelius, A.; Pestalozzi, B.C.; Knuth, A. Molecular targeted therapy. Der Chirurg 2006, 77, 1118–1125. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Panda, D.; Jordan, M.A. Modulation of microtubule dynamics by drugs: A paradigm for the actions of cellular regulators. Cell Struct. Funct. 1999, 24, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Ann. Rev. Cell Dev. Biol. 2000, 16, 89–111. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Song, Y.; Iskander, M.N. Investigation of structural requirements of anticancer activity at the paclitaxel/tubulin binding site using comfa and comsia. J. Mol. Graph. Model. 2003, 21, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Desbene, S.; Giorgi-Renault, S. Drugs that inhibit tubulin polymerization: The particular case of podophyllotoxin and analogues. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 71–90. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Renuka, B.; Kumar, G.S.; Vandana, V.; Deevi, D.S. 9-Deoxopodophyllotoxin derivatives as anti-cancer agents. Bioorg. Med. Chem. Lett. 1999, 9, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.B.; You, Y.J.; Ahn, B.Z. Deoxypodophyllotoxin; the cytotoxic and antiangiogenic component from pulsatilla koreana. Planta Med. 2002, 68, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, C.; Yamamoto, N.; Nakao, H.; Toi, Y.; Chikahisa-Muramatsu, L.; Kanemaru, K.; Masuda, T.; Oyama, Y. Flow cytometric estimation of cytotoxic activity of rhodexin A isolated from rhodea japonica in human leukemia K562 cells. Biol. Pharm. Bull. 2003, 26, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Antitumor activity of deoxypodophyllotoxin isolated from Anthriscus sylvestris: Induction of G2/M cell cycle arrest and caspase-dependent apoptosis. Bioorg. Med. Chem. Lett. 2009, 19, 4367–4371. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Kim, J.R.; Jin, U.H.; Choi, H.S.; Chang, Y.C.; Lee, Y.C.; Kim, S.H.; Lee, I.S.; Moon, T.C.; Chang, H.W.; et al. Deoxypodophyllotoxin, flavolignan, from Anthriscus sylvestris Hoffm. Inhibits migration and MMP-9 via MAPK pathways in TNF-alpha-induced HASMC. Vasc. Pharmacol. 2009, 51, 13–20. [Google Scholar] [CrossRef]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Widrow, R.J.; Rabinovitch, P.S.; Cho, K.; Laird, C.D. Separation of cells at different times within G2 and mitosis by cyclin B1 flow cytometry. Cytometry 1997, 27, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Grana, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Park, H.Y.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef] [PubMed]
- Czekierdowski, A.; Czekierdowska, S.; Czuba, B.; Cnota, W.; Sodowski, K.; Kotarski, J.; Zwirska-Korczala, K. Microvessel density assessment in benign and malignant endometrial changes. J. Physiol. Pharmacol. 2008, 59, 45–51. [Google Scholar] [PubMed]
- Nagy, V.M.; Buiga, R.; Brie, I.; Todor, N.; Tudoran, O.; Ordeanu, C.; Virag, P.; Tarta, O.; Rus, M.; Balacescu, O. Expression of VEGF, VEGFR, EGFR, COX-2 and mvd in cervical carcinoma, in relation with the response to radio-chemotherapy. Roman. J. Morphol. Embryol. 2011, 52, 53–59. [Google Scholar]
- Folkman, J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann. Surg. 1972, 175, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Honore, S.; Braguer, D. Microtubule-targeting agents in angiogenesis: Where do we stand? Drug Resist. Updates 2006, 9, 74–86. [Google Scholar] [CrossRef]
- Schwartz, E.L. Antivascular actions of microtubule-binding drugs. Clin. Cancer Res. 2009, 15, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, X.; Xu, F.; Xu, B.; He, X.; Huang, B.; Yuan, M. Synthesis and cytotoxic activity evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Molecules 2014, 19, 12048–12064. [Google Scholar] [CrossRef] [PubMed]
- Lieuvin, A.; Labbe, J.C.; Doree, M.; Job, D. Intrinsic microtubule stability in interphase cells. J. Cell Biol. 1994, 124, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, H.; Yan, L.; Tian, S.; Li, D.; Xu, Z. Microvessel density and heparanase over-expression in clear cell renal cell cancer: Correlations and prognostic significances. World J. Surg. Oncol. 2011, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.J.; Cheng, X.G.; Qu, H.; Shen, B.Z.; Han, M.J.; Wu, Z.H. Solitary pulmonary nodules: Comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density. Chin. Med. J. 2009, 122, 541–547. [Google Scholar] [PubMed]
- Sample Availability: Samples are available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-R.; Xu, Y.; Jiang, Z.-Z.; Guerram, M.; Wang, B.; Zhu, X.; Zhang, L.-Y. Deoxypodophyllotoxin Induces G2/M Cell Cycle Arrest and Apoptosis in SGC-7901 Cells and Inhibits Tumor Growth in Vivo. Molecules 2015, 20, 1661-1675. https://doi.org/10.3390/molecules20011661
Wang Y-R, Xu Y, Jiang Z-Z, Guerram M, Wang B, Zhu X, Zhang L-Y. Deoxypodophyllotoxin Induces G2/M Cell Cycle Arrest and Apoptosis in SGC-7901 Cells and Inhibits Tumor Growth in Vivo. Molecules. 2015; 20(1):1661-1675. https://doi.org/10.3390/molecules20011661
Chicago/Turabian StyleWang, Yu-Rong, Yuan Xu, Zhen-Zhou Jiang, Mounia Guerram, Bin Wang, Xiong Zhu, and Lu-Yong Zhang. 2015. "Deoxypodophyllotoxin Induces G2/M Cell Cycle Arrest and Apoptosis in SGC-7901 Cells and Inhibits Tumor Growth in Vivo" Molecules 20, no. 1: 1661-1675. https://doi.org/10.3390/molecules20011661





