Xanthones from the Roots of Moutabea guianensis Aubl.
Abstract
:1. Introduction
2. Results and Discussion

| R1 | R2 | R3 | |
|---|---|---|---|
| 1 | H | H | OH |
| 2 | OCH3 | OCH3 | OH |
| 3 | OCH3 | H | OH |
| 4 | H | OCH3 | OH |
| 5 | OCH3 | OCH3 | OCH3 |
| Positions | Compound 2 | Compound 5 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC and DEPT | δH (J in Hz) | δC and DEPT | |
| 1 | 154.1, C | 154.9, C | ||
| 2 | 6.73, d, 9.0 | 109.3, CH | 6.70, d, 8.8 | 109.0, CH |
| 3 | 7.26, d, 9.0 | 121.3, CH | 7.22, d, 8.8 | 120.4, CH |
| 4 | 140.3 | 139.8, C | ||
| 4a | 145.6, C * | 145.1, C | ||
| 4b | 145.8, C * | 147.7, C | ||
| 5 | 132.8, C | 137.4, C | ||
| 6 | 154.9, C | 153.1, C | ||
| 7 | 135.7, C | 149.2, C | ||
| 8 | 150.2, C | 143.2, C | ||
| 8a | 103.9, C | 111.2, C | ||
| 8b | 107.8, C | 109.4, C | ||
| 9 | 185.5, C | 181.6, C | ||
| 4-OCH3 | 3.97, s | 57.6, CH3 | 3.96, s | 57.6 , CH3 |
| 5-OCH3 | 4.00, s | 61.7, CH3 | 4.10, s | 61.9, CH3 |
| 6-OCH3 | 4.16, s | 61.9, CH3 | 4.14, s | 61.7, CH3 |
| 7-OCH3 | 3.94, s | 61.2, CH3 | 3.99, s | 62.1, CH3 |
| 8-OCH3 | 3.94, s | 62.8, CH3 | ||
| 1-OH | 11.29, s | 12.39, s | ||
| 8-OH | 11.77, s | |||
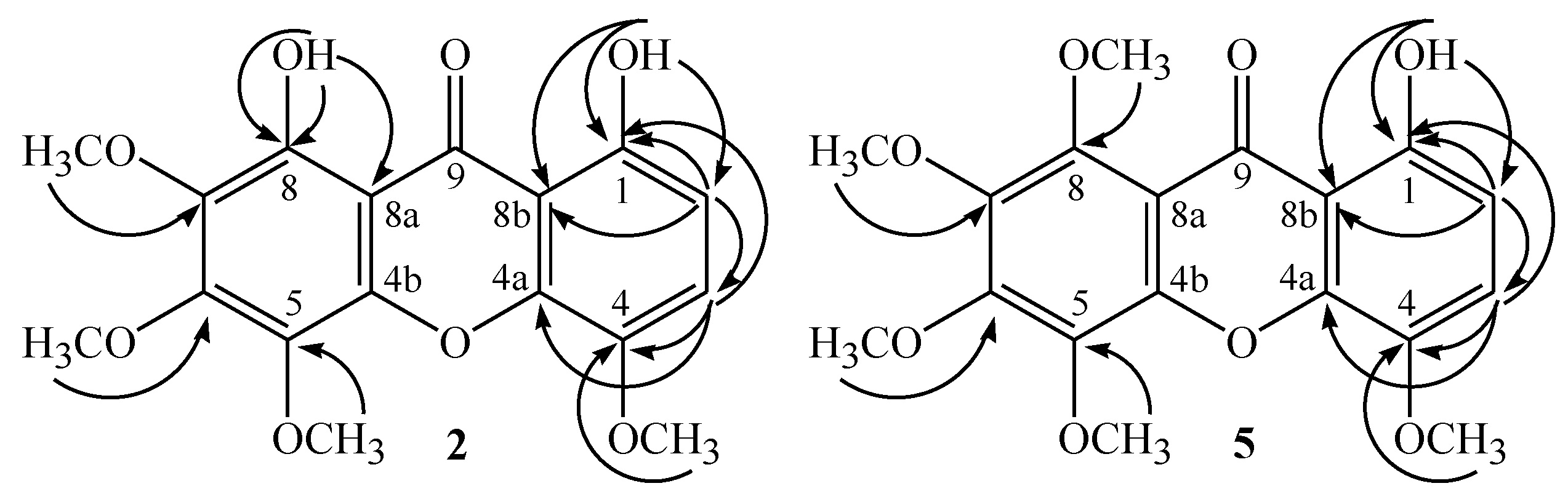
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization
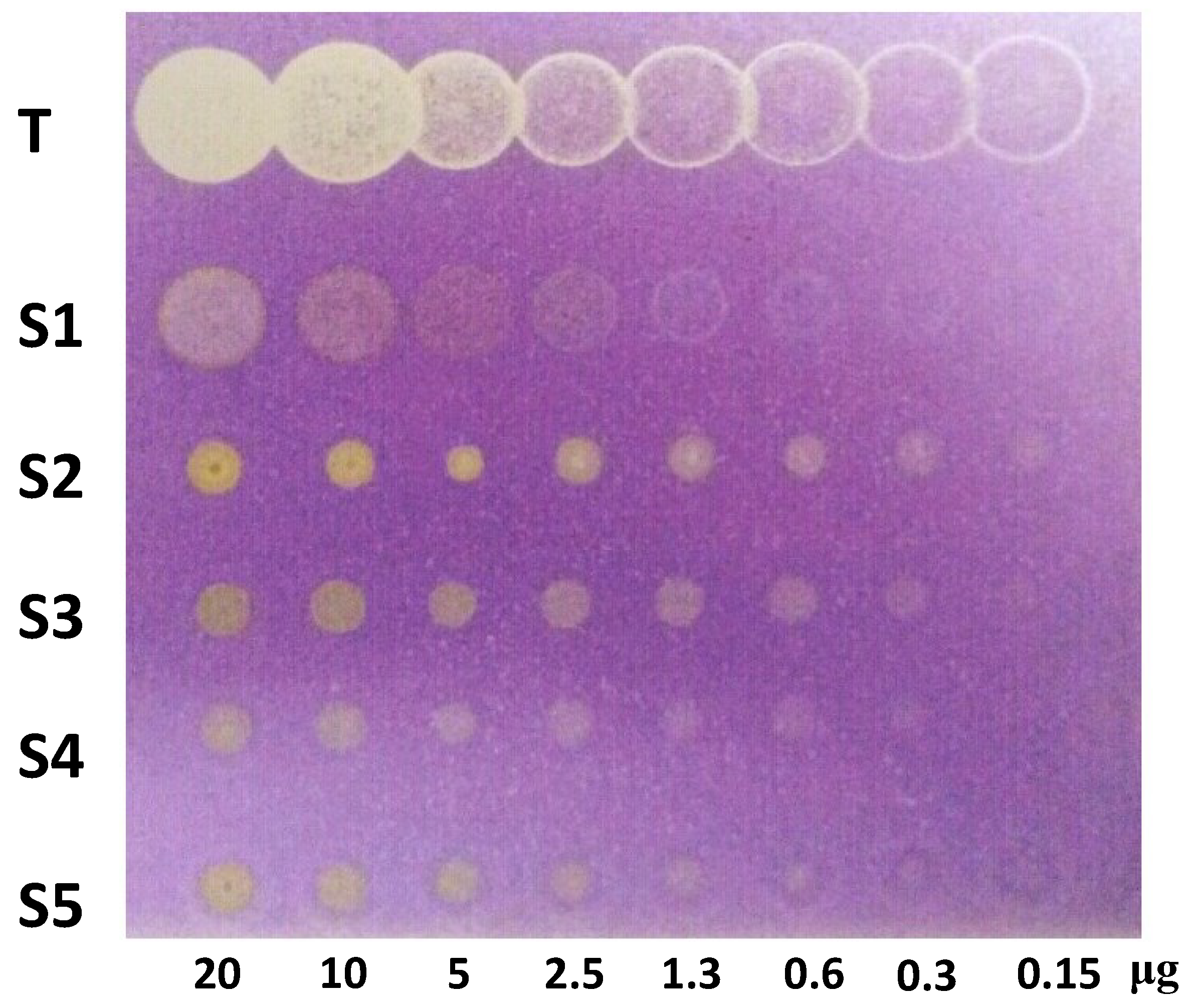
3.5. Antioxidant Capacity by DPPH Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cardona, M.L.; Fernándes, I.; Pedro, J.R.; Serrano, A. Xanthones from Hypericum reflexum. Phytochemistry 1990, 29, 3003–3006. [Google Scholar] [CrossRef]
- Rocha, J.L.C.; Pastore, J.F.B.; Brandão, H.N.; Azevedo, A.; David, J.P.; Santos, E.O.; David, J.M. Quantificação de salicilato de metila em quatro gêneros de Polygalaceae, por CLAE-DAD. Quim. Nova 2012, 35, 2263–2266. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J. Naturally occurring pentaoxygenated, hexaoxyganated and dimeric xanthones: A literature survey. Quim. Nova 1997, 20, 388–397. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; Oliveira, F.F. Tetraoxygenated naturally occuring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef] [PubMed]
- Dibwe, D.F.; Awale, S.; Kadota, S.; Morita, H.; Tezuka, Y. Muchimangins E and F: Novel diphenylmethyl-substituted xanthones from Securidaca longepedunculata. Tetrahedron Lett. 2014, 55, 1916–1919. [Google Scholar] [CrossRef]
- Ripardo Filho, H.S.; Pacheco, L.C.; da S. Andrade, E.; Correa, M.J.C.; Guilhon, G.M.S.P.; Santos, L.S. A new xanthone from Moutabea guianensis Aubl. Molecules 2014, 19, 8885–8889. [Google Scholar] [CrossRef]
- Astana, R.K.; Sharma, N.K.; Kulshreshtha, D.K.; Chatterjee, S.K.A. Xanthone from Swertia. chirayata. Phytochemistry 1991, 30, 1037–1039. [Google Scholar] [CrossRef]
- Benn, M.H.; Joyce, N.I.; Lorimer, S.D.; Perry, N.B.; van Klink, J.W.; Wu, Q. Xanthones and bisxanthones in five New Zealand and subantarctic Gentianella species. Biochem. Syst. Ecol. 2009, 37, 531–534. [Google Scholar]
- Zhu, B.; Zhe, W.; Duan, Y.; Wang, M.; Gao, Y.; Wei, G.; Liao, T. Two new xanthones from Swertia angustifolia. J. Asian Nat. Prod. Res. 2012, 14, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, W.G.; Labadie, R.P. Polyoxygenated xanthones of Centaurium littorale. Phytochemistry 1985, 24, 2601–2605. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Rastogi, R.P. 13C NMR spectroscopy of flavonoids. Heterocycles 1981, 16, 2181–2236. [Google Scholar] [CrossRef]
- Marston, A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A 2011, 1218, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Mahabusarakam, W; Proudfoot, J.; Taylor, W.; Croft, K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radical Res. 2000, 33, 643–659. [Google Scholar] [CrossRef]
- Blanco-Ayala, T.; Lugo-Huitrón, R.; Serrano-López, E.M.; Reyes-Chilpa, R.; Rangel-López, E.; Pineda, B.; Medina-Campos, O.N.; Sánchez-Chapul, L.; Pinzón, E.; Cristina, T.-S.; et al. Antioxidant properties of xanthones from Calophyllum brasiliense: Prevention of oxidative damage induced by FeSO4. BMC Complement. Altern. Med. 2013, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawamoto, A.; Tamura, A.; Tatsumi, Y.; Fujii, T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid). Chem. Pharm. Bull. 1992, 40, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Benabadji, S.H.; Wen, R.; Zheng, J.B.; Dong, X.C.; Yuan, S.G. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol. Sin. 2004, 25, 666–671. [Google Scholar] [PubMed]
- Gu, L.; Wua, T.; Wang, Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT-Food Sci. Technol. 2009, 42, 131–136. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound 2 is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, H.D.S.R.; Pacheco, L.C.; Andrade, E.D.S.; Correa, M.J.C.; Araújo, R.N.M.; Guilhon, G.M.S.P.; Silva, J.K.R.d.; Santos, L.S. Xanthones from the Roots of Moutabea guianensis Aubl. Molecules 2015, 20, 127-134. https://doi.org/10.3390/molecules20010127
Filho HDSR, Pacheco LC, Andrade EDS, Correa MJC, Araújo RNM, Guilhon GMSP, Silva JKRd, Santos LS. Xanthones from the Roots of Moutabea guianensis Aubl. Molecules. 2015; 20(1):127-134. https://doi.org/10.3390/molecules20010127
Chicago/Turabian StyleFilho, Haroldo Da S. Ripardo, Luidi C. Pacheco, Edinaldo Da S. Andrade, Marivaldo José C. Correa, Railda Neyva M. Araújo, Giselle Maria S. P. Guilhon, Joyce Kelly R. da Silva, and Lourivaldo S. Santos. 2015. "Xanthones from the Roots of Moutabea guianensis Aubl." Molecules 20, no. 1: 127-134. https://doi.org/10.3390/molecules20010127






