Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables
Abstract
:1. Introduction
2. Results and Discussion
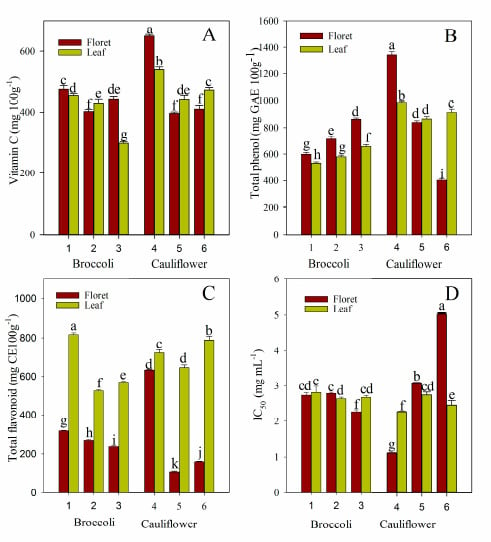
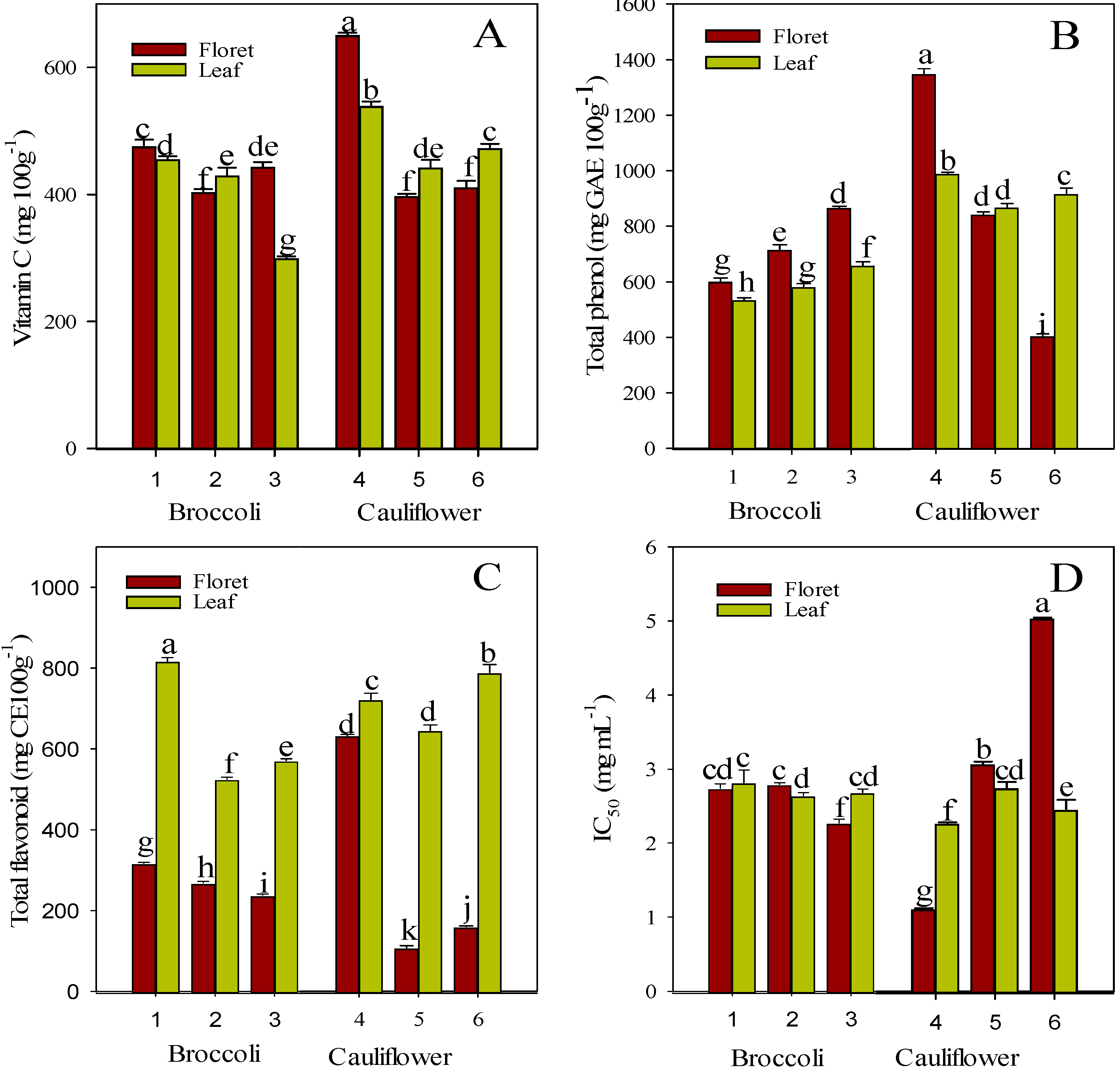
2.1. Vitamin C, Total Phenol, and Total Flavonoid Content
2.2. Glucosinolate Profiles
| Crop | Cultivar | Part | Progoitrin | Glucoraphanin | Sinigrin | Gluconapin | Glucobrassicanapin | Glucobrassicin | Total Glucosinolates |
|---|---|---|---|---|---|---|---|---|---|
| Broccoli | AMaGi | Floret | 2.73 x a y | 2.77 b | N/D z | 0.56 a | N/D | 2.03 c | 8.09 c |
| Leaf | 0.34 e | 2.05 d | N/D | 0.20 c | N/D | 2.30 c | 4.89 fg | ||
| Baeridom | Floret | 1.66 b | 5.19 a | 0.31 e | 0.43 b | N/D | 2.07 c | 9.66 a | |
| Leaf | 0.22 f | 2.11 d | 0.06 f | 0.08 de | N/D | 1.93 c | 4.40 g | ||
| Cheonjae | Floret | 0.44 d | 2.50 c | 0.09 f | 0.12 d | N/D | 2.44 c | 5.59 ef | |
| Leaf | 0.04 h | 1.10 e | 0.02 f | 0.04 ef | N/D | 0.92 d | 2.12 hi | ||
| Cauliflower | Asia purple | Floret | 0.41 d | 0.69 fg | 1.71 a | N/D | 0.85 a | 5.14 a | 8.80 b |
| Leaf | 0.13 g | 0.32 h | 1.04 c | N/D | 0.43 b | 5.28 a | 7.20 d | ||
| Asia white | Floret | 0.51 c | 0.65 fg | 0.12 f | 0.59 a | 0.05 c | 0.05 e | 1.97 h | |
| Leaf | 0.07 h | 0.73 fg | 0.44 d | N/D | 0.42 b | 1.15 d | 2.81 h | ||
| Bridal | Floret | 0.31 e | 0.76 f | 1.17 b | 0.09 de | N/D | 5.08 a | 7.41 cd | |
| Leaf | 0.16 g | 0.49 gh | 0.98 c | 0.11 d | 0.41 b | 3.69 b | 5.84 e |
2.3. Free Sugar Content
| Crop | Cultivar | Part | Glucose | Fructose | Sucrose | Total Free Sugar |
|---|---|---|---|---|---|---|
| Broccoli | AMaGi | Floret | 77.4 x g y | 75.3 f | 0.6 j | 153.3 h |
| Leaf | 134.5 b | 99.3 d | 7.8 g | 241.6 d | ||
| Baeridom | Floret | 105.5 f | 98.4 d | 1.9 i | 205.8 f | |
| Leaf | 112.8 ef | 78.2 f | 23.3 d | 214.3 ef | ||
| Cheonjae | Floret | 126.3 b–d | 110.2 b | 0.7 j | 237.2 d | |
| Leaf | 118.9 de | 100.8 cd | 4.0 h | 223.7 e | ||
| Cauliflower | Asia purple | Floret | 133.3 b | 104.1 c | 32.2 b | 269.6c |
| Leaf | 127.8 bc | 49.4 h | 14.8 e | 192.0 g | ||
| Asia white | Floret | 161.8 a | 127.0 a | 10.5 f | 299.3 b | |
| Leaf | 120.5 c–e | 65.1 g | 29.2 c | 214.8 ef | ||
| Bridal | Floret | 133.1 b | 113.5 b | 72.0 a | 318.6 a | |
| Leaf | 127.3 bc | 88.3 e | 8.8 g | 224.4 e |
2.4. Fatty Acid Composition
| Fatty acids | Broccoli | Cauliflower | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMaGi | Baeridom | Cheonjae | Asia purple | Asia white | Bridal | |||||||
| Floret | Leaf | Floret | Leaf | Floret | Leaf | Floret | Leaf | Floret | Leaf | Floret | Leaf | |
| Lauric (C12:0) | 0.17 u a v | N/D w | 0.10 b | N/D | 0.10 b | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Myristic (C14:0) | 1.04 a | 0.39 f | 0.51 e | 0.28 g | 0.39 f | 0.29 g | 0.87 b | 0.91 b | 0.87 b | 0.68 d | 0.77 c | 0.67 d |
| Pentadecanoic (C15:0) | 0.40 ab | 0.44 a | 0.29 e | 0.38 bc | 0.35 cd | 0.36 b–d | 0.37 bc | 0.30 e | 0.36 b–d | 0.37 bc | 0.32 de | 0.44 a |
| Palmitic (C16:0) | 31.74 c | 23.55 g | 29.75 e | 24.05 g | 31.03 cd | 23.52 g | 30.50 de | 27.11 f | 34.75 b | 27.25 f | 38.42 a | 27.15 f |
| Palmitoleic (C16:1) | 0.66 a | 0.21 de | 0.44 c | 0.21 de | 0.37 c | 0.13 e | 0.73 a | 0.22 d | 0.53 b | 0.17 de | 0.40 c | 0.20 de |
| Heptadecanoic (C17:0) | 0.61 a | 0.40 e | 0.30 fg | 0.33 f | 0.31 fg | 0.27 g | 0.59 a | 0.47 bc | 0.51 b | 0.45 cd | 0.41 de | 0.42 de |
| Stearic (C18:0) | 11.90 a | 5.53 g | 5.98 f | 4.70 i | 5.59 g | 5.07 h | 10.37 b | 11.72 a | 9.97 d | 10.11 cd | 8.73 e | 10.24 bc |
| Oleic (C18:1n9c) | 3.63 d–g | 3.13 g | 3.82 b–e | 3.22 fg | 4.30 ab | 3.74 c–f | 3.56 e–g | 3.99 b–e | 4.12 a–d | 4.23 a–c | 4.61 a | 3.75 c–f |
| Linoleic (C18:2n6c) | 13.56 ef | 14.43 d | 18.02 b | 14.09 de | 18.23 b | 14.36 d | 16.42 c | 14.38 d | 16.69 c | 14.52 d | 18.97 a | 13.09 f |
| Linolenic (C18:3n3) | 34.34 f | 50.72 a | 39.13 de | 51.80 a | 37.80 e | 51.03 a | 35.52 f | 39.76 cd | 30.61 g | 41.00 c | 26.32 h | 42.76 b |
| Arachidic (C20:0) | 0.99 a | 0.53 de | 0.84 ab | 0.42 e | 0.74 a–d | 0.44 e | 0.48 e | 0.55 e | 0.81 a–c | 0.57 c–e | 0.61 b–e | 0.62 b–e |
| Behenic (C22:0) | 0.30 a | 0.21 a–c | 0.22 a–c | 0.12 c | 0.22 a–c | 0.28 ab | 0.22 a–c | 0.23 a–c | 0.32 a | 0.21 a–c | 0.17 b–c | 0.23 a–c |
| Lignoceric (C24:0) | 0.66 a | 0.46 cd | 0.60 ab | 0.40 cd | 0.57 ab | 0.51 bc | 0.37 de | 0.36 de | 0.46 cd | 0.44 cd | 0.27 e | 0.43 cd |
| SFA x | 47.81 b | 31.51 g | 38.59 f | 30.68 g | 39.30 e | 30.74 g | 43.77 c | 41.65 d | 48.05 b | 40.08 e | 49.70 a | 40.20 e |
| MUFA y | 4.29 bc | 3.34 e | 4.26 bc | 3.43 dc | 4.67 ab | 3.87 cd | 4.29 bc | 4.21 bc | 4.65 ab | 4.40 bc | 5.01 a | 3.95 c |
| PUFA z | 47.90 f | 65.15 a | 57.15 b | 65.89 a | 56.03 c | 65.39 a | 51.94 e | 54.14 d | 47.30 f | 55.52 c | 45.29 g | 55.85 c |
2.5. Antioxidant Activity
2.6. Correlations among Phytonutrients
| Attributes | Total Phenol | Total Flavonoid | Total Glucosinolate | Antioxidant Activity |
|---|---|---|---|---|
| Vitamin C | 0.522 ** | 0.574 ** | 0.494 ** | 0.632 ** |
| Total phenol | 1 | 0.292 | 0.169 | 0.698 ** |
| Total flavonoid | 0.292 | 1 | 0.014 | 0.456 ** |
| Total glucosinolate | 0.169 | 0.014 | 1 | 0.219 |
3. Experimental Section
3.1. Plant Materials
3.2. Authentic Standards and Chemicals
3.3. Analysis of Vitamin C
3.4. Analysis of Total Phenol
3.5. Analysis of Total Flavonoid
3.6. Analysis of Glucosinolates
3.7. Analysis of Free Sugar
3.8. Analysis of Fatty Acid Composition
3.9. Determination of Antioxidant Activity
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Byers, T.; Perry, G. Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers. Annu. Rev. Nutr. 1992, 12, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on Brassica vegetables and cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 733–748. [Google Scholar]
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2012, 5, 260–266. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A.S. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important Brassica vegetables. Molecules 2011, 16, 6816–6832. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.A.; Jeffery, E.H.; Wallig, M.A.; Klein, B.P. Retention of phytochemicals in fresh and processed broccoli. J. Food Sci. 1997, 62, 1098–1100. [Google Scholar] [CrossRef]
- Bellostas, N.; Sorensen, J.C.; Sorensen, H. Qualitative and quantitative evaluation of glucosinolates in cruciferous plants during their life cycle. Agroindustria 2004, 3, 5–10. [Google Scholar]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Campbell, B.; Han, D.Y.; Triggs, C.M.; Fraser, A.G.; Ferguson, L.R. Brassicaceae: Nutrient analysis and investigation of tolerability in people with Crohn’s disease in a New Zealand study. Funct. Foods Health Dis. 2012, 2, 460–486. [Google Scholar]
- Razis, A.F.A.; Noor, N.M. Cruceferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Graser, G.; Schneider, B.; Oldham, N.J.; Gershenzon, J. The methionine chain elongation pathway in the biosynthesis of glucosinolates in Eruca sativa (Brassicaceae). Arch. Biochem. Biophys. 2000, 378, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, A.W.; Oey, I.; Loey, A.V.; Hendrickx, M. Application of thermal inactivation of enzymes during vitamin C analysis to study the influence of acidification, crushing and blanching on vitamin C stability in broccoli (Brassica oleracea L var. italica). Food Chem. 2010, 120, 591–598. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Ciska, E.; Pawlak-Lemanska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Schonhof, I.; Krumbein, A.; Bruckner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Nahrung 2004, 48, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, R.L.; Etherton, T.D.; Pearson, T.A.; Harrison, E.H.; Kris-Etherton, M. Low fat and high monounsaturated fat diets decrease human low density lipoprotein oxidative susceptibility in vitro. J. Nutr. 2001, 131, 1758–1763. [Google Scholar] [PubMed]
- Iso, H.; Sato, S.; Umemura, U.; Kudo, M.; Koike, K.; Kitamura, A.; Imano, H.; Okamura, T.; Naito, Y.; Shimamoto, T. Linoleic acid, other fatty acids, and risk of stroke. Stroke 2002, 33, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Kwak, J.H. Seasonal variation in phytochemicals and antioxidant activities in different tissues of various Broccoli cultivars. Afr. J. Biotechnol. 2014, 13, 604–615. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Comp. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Samec, D.; Piljac-Zegarac, J.; Bogovic, M.; Habjanic, K.; Gruz, J. Antioxidant potency of white (Brassica oleracea L. var. capitata) and Chinese (Brassica rapa L. var. pekinensis (Lour.)) cabbage: The influence of development stage, cultivar choice and seed selection. Sci. Hortic. 2011, 128, 78–83. [Google Scholar]
- Scalzo, R.L.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant properties and lipidic profile as quality indexes of cauliflower (Brassica oleracea L. var. botrytis) in relation to harvest time. Food Chem. 2007, 100, 1019–1025. [Google Scholar]
- Rosa, E.; David, M.; Gomes, M.H. Glucose, fructose and sucrose content in broccoli, white cabbage and Portuguese cabbage grown in early and late seasons. J. Sci. Food Agric. 2001, 81, 1145–1149. [Google Scholar] [CrossRef]
- Lee, J.G.; Kwak, J.H.; Um, Y.C.; Lee, S.G.; Jang, Y.A.; Choi, C.S. Variation of glucosinolate contents among domestic broccoli (Brassica oleracea L. var. italica) accessions. Korean J. Hortic. Sci. Technol. 2012, 30, 743–750. [Google Scholar] [CrossRef]
- Velasco, L.; Fernando, D.G.; Becker, H.C. Variability for the fatty acid composition of the seed oil in a germplasm collection of the genus Brassica. Genet. Res. Crop Evol. 1998, 45, 371–382. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Comp. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Picchi, V.; Migliori, C.; Lo Scalzo, R.; Campanelli, G.; Ferrari, V.; Di Cesare, L.F. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chem. 2012, 130, 501–509. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Rosa, E.A.S.; Rodrigues, A.S. Total and individual glucosinolate contents in 11 broccoli cultivars grown in early and late seasons. HortScience 2001, 36, 56–59. [Google Scholar]
- Hodges, D.M.; Munro, K.D.; Forney, C.F.; McRae, K.B. Glucosinolate and free sugar content in cauliflower (Brassica oleracea var. botrytis cv. Freemont) during controlled-atmosphere storage. Postharvest Biol. Technol. 2006, 40, 123–132. [Google Scholar]
- Kim, K.S.; Park, S.H.; Choung, M.G. Nondestructive determination of oil content and fatty acid composition in perilla seeds by near-infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Pomeroy, S.E.; Sasahara, T.; Yamashita, T.; Liang, Y.L.; Dart, A.M.; Jennings, G.L.; Abbey, M.; Cameron, J.D. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Samec, D.; Bogovic, M.; Vincek, D.; Martincic, J.; Salopek-Sondi, B. Assessing the authenticity of the white cabbage (Brassica oleracea var. capitata f. alba) cv. “Varaždinski” by molecular and phytochemical markers. Food Res. Int. 2014, 60, 266–272. [Google Scholar]
- Spinola, V.; Mendes, B.; Camara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetric of total phenols with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lee, J.G.; Bonnema, G.; Zhang, N.; Kwak, J.H.; de Vos, R.C.; Beekwilder, J. Evaluation of glucosinolate variation in a collection of turnip (Brassica rapa) germplasm by the analysis of intact and desulfo glucosinolates. J. Agric. Food Chem. 2013, 61, 3984–3993. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Basnet, S.; Chung, K.H.; Ryu, K.H.; Lee, Y.S. Comparisons of nutritional and phytochemical property of genetically modified CMV-resistant red pepper and its parental cultivar. Hortic. Environ. Biotechnol. 2012, 53, 151–157. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.R.; Kwak, J.-H. Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables. Molecules 2015, 20, 1228-1243. https://doi.org/10.3390/molecules20011228
Bhandari SR, Kwak J-H. Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables. Molecules. 2015; 20(1):1228-1243. https://doi.org/10.3390/molecules20011228
Chicago/Turabian StyleBhandari, Shiva Ram, and Jung-Ho Kwak. 2015. "Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables" Molecules 20, no. 1: 1228-1243. https://doi.org/10.3390/molecules20011228