An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma lucidum and Its Five Related Species Originating from Vietnam
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Preparation Condition
2.2. Selection of HPLC Conditions and Validation of the Developed Method
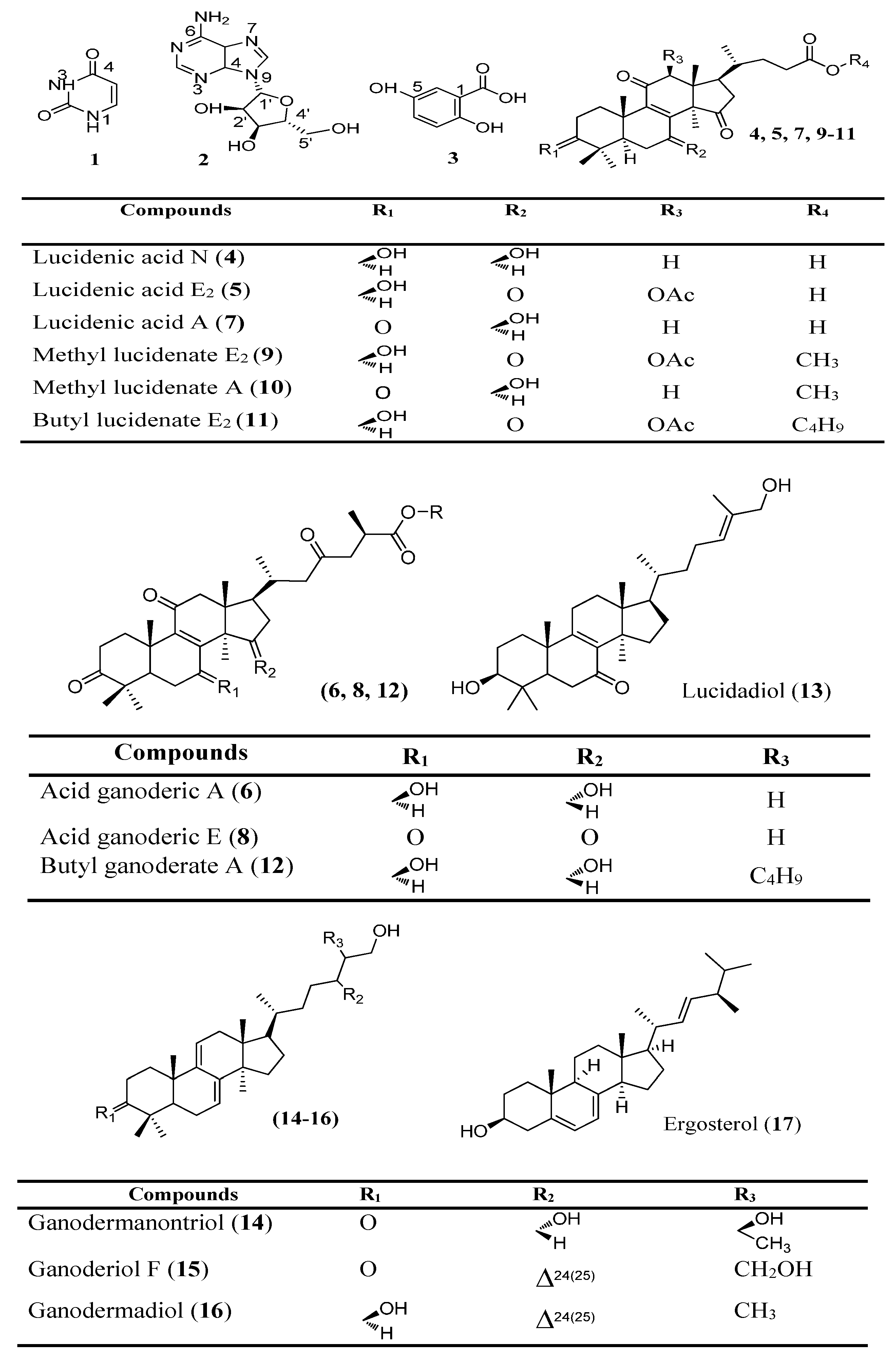
| Compounds | Retention Time Reproducibility (min, n = 8) | Regression Equation | r2 | *F | *p (F) | Linearity Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|---|---|
| Acid | ||||||||
| Lucidenic acid N (4) | 48.176 ± 0.110 | y = 20041.5x − 11913.8 | 0.9999 | 12.67 | 0.00314 | 7.50–180.00 | 0.88 | 2.64 |
| Lucidenic acid E2 (5) | 56.463 ± 0.161 | y = 10679.7x − 4195.8 | 0.9999 | 12.74 | 0.00308 | 7.50–180.00 | 0.82 | 2.45 |
| Ganoderic acid A (6) & Lucidenic acid A (7) | 74.075 ± 0.130 | y = 19900.9x − 11310.7 | 0.9999 | 12.77 | 0.00305 | 7.50–180.00 | 0.34 | 1.01 |
| Ganoderic acid E (8) | 83.898 ± 0.240 | y = 5790.0x − 4699.3 | 0.9999 | 12.60 | 0.00320 | 7.50–180.00 | 1.17 | 3.52 |
| Methyl lucidenate E2 (9) | 93.330 ± 0.083 | y = 6087.3x − 5267.7 | 0.9999 | 12.59 | 0.00321 | 7.50–180.00 | 0.91 | 2.74 |
| Methyl lucidenate A (10) | 97.480 ± 0.080 | y = 31602.7x − 12081.8 | 0.9999 | 12.74 | 0.00308 | 7.50–180.00 | 0.68 | 2.04 |
| Butyl lucidenate E2 (11) & Butyl ganoderate A (12) | 101.827 ± 0.116 | y = 9737.0x − 12899.6 | 0.9999 | 12.65 | 0.00316 | 7.50–180.00 | 1.10 | 3.31 |
| Alcohol | ||||||||
| Lucidadiol (13) | 104.729 ± 0.115 | y = 9573.6x − 3631.9 | 0.9999 | 12.74 | 0.00308 | 7.50–180.00 | 0.57 | 1.72 |
| Ganodemanontriol (14) | 105.589 ± 0.213 | y = 34791.4x + 110902.3 | 0.9999 | 13.89 | 0.00226 | 7.50–180.00 | 1.39 | 4.18 |
| Ganoderiol F (15) | 111.230 ± 0.245 | y = 7417.9x − 14493.5 | 0.9999 | 12.25 | 0.00353 | 7.50–180.00 | 1.41 | 4.23 |
| Ganodermadiol (16) | 121.772 ± 0.258 | y = 11250.1x − 5594.5 | 0.9999 | 12.70 | 0.00311 | 7.50–180.00 | 1.37 | 4.12 |
| Other | ||||||||
| Ergosterol (17) | 128.915 ± 0.267 | y = 18821.0x − 5702.9 | 0.9999 | 12.77 | 0.00306 | 7.50–180.00 | 0.94 | 2.82 |
| Compounds | Added (µg/mL) | Detected (µg/mL) | Recovery (%) | M ± SD (%) | RSD (%) |
|---|---|---|---|---|---|
| Acid | |||||
| Lucidenic acid N (4) | 9.50 | 9.47 | 99.68 | 99.51 ± 1.48 | 1.49 |
| 19.00 | 19.17 | 100.89 | |||
| 38.00 | 37.22 | 97.95 | |||
| Lucidenic acid E2 (5) | 10.20 | 10.23 | 100.29 | 98.44 ± 1.61 | 1.64 |
| 20.40 | 19.92 | 97.65 | |||
| 40.80 | 39.73 | 97.38 | |||
| Ganoderic acid A (6) & Lucidenic acid A (7) | 10.40 | 10.21 | 98.17 | 98.71 ± 1.23 | 1.25 |
| 20.80 | 20.35 | 97.84 | |||
| 41.60 | 41.65 | 100.12 | |||
| Ganoderic acid E (8) | 10.80 | 10.54 | 97.59 | 99.20 ± 1.45 | 1.46 |
| 21.60 | 21.69 | 100.42 | |||
| 43.20 | 43.02 | 99.58 | |||
| Methyl lucidenate E2 (9) | 10.00 | 10.28 | 102.80 | 100.79 ± 1.74 | 1.73 |
| 20.00 | 19.95 | 99.75 | |||
| 40.00 | 39.93 | 99.83 | |||
| Methyl lucidenate A (10) | 10.10 | 9.82 | 97.23 | 98.39 ± 1.76 | 1.79 |
| 20.20 | 19.70 | 97.52 | |||
| 40.40 | 40.57 | 100.42 | |||
| Butyl lucidenate E2 (11) and Butyl ganoderate A (12) | 10.10 | 9.93 | 98.32 | 97.52 ± 1.95 | 2.00 |
| 20.20 | 19.25 | 95.30 | |||
| 40.40 | 39.97 | 98.94 | |||
| Alcohol | |||||
| Lucidadiol (13) | 10.50 | 10.12 | 96.38 | 97.17 ± 2.28 | 2.35 |
| 21.00 | 20.03 | 95.38 | |||
| 42.00 | 41.89 | 99.74 | |||
| Ganodemanontriol (14) | 10.20 | 10.12 | 99.22 | 99.32 ± 1.27 | 1.27 |
| 20.40 | 20.53 | 100.64 | |||
| 40.80 | 40.03 | 98.11 | |||
| Ganoderiol F (15) | 10.40 | 10.05 | 96.63 | 97.09 ± 1.12 | 1.15 |
| 20.80 | 20.46 | 98.37 | |||
| 41.60 | 40.05 | 96.27 | |||
| Ganodermadiol (16) | 10.00 | 9.94 | 99.40 | 99.45 ± 1.63 | 1.63 |
| 20.00 | 20.22 | 101.10 | |||
| 40.00 | 39.14 | 97.85 | |||
| Other | |||||
| Ergosterol (17) | 10.40 | 10.07 | 96.83 | 97.70 ± 2.21 | 2.26 |
| 20.80 | 19.98 | 96.06 | |||
| 41.60 | 41.69 | 100.22 | |||
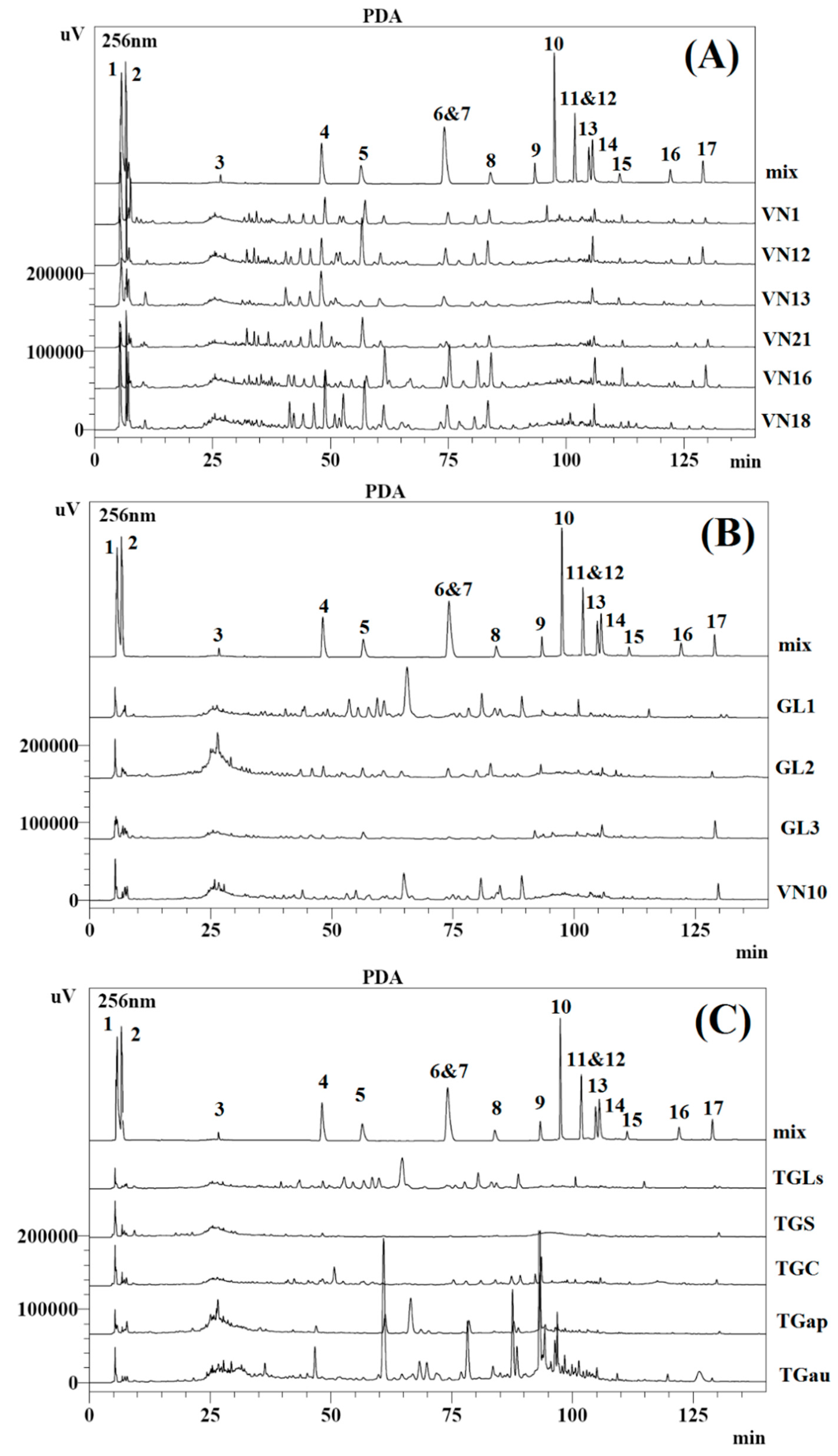
2.3. Quantitative Comparison of Different Ganoderma Species
| Compounds | Nominal Conc. (µg/mL) | Inter-Day | Intra-Day | ||||
|---|---|---|---|---|---|---|---|
| Observed Conc. (M ± SD), (μg/mL) | Accuracy (%) | Precision (%) | Observed Conc. (M ± SD), (μg/mL) | Accuracy (%) | Precision (%) | ||
| Lucidenic acid N (4) | 30.25 | 30.02 ± 0.13 | 99.24 | 0.43 | 30.17 ± 0.42 | 99.74 | 1.39 |
| 60.50 | 60.13 ± 2.10 | 99.39 | 3.49 | 61.02 ± 1.95 | 100.86 | 3.20 | |
| 121.00 | 120.04 ± 2.29 | 99.21 | 1.91 | 120.11 ± 2.25 | 99.26 | 1.87 | |
| Lucidenic acid E2 (5) | 29.75 | 30.33 ± 0.12 | 101.95 | 0.40 | 30.12 ± 0.50 | 101.24 | 1.66 |
| 59.50 | 60.17 ± 0.97 | 101.13 | 1.61 | 58.27 ± 0.79 | 97.93 | 1.36 | |
| 119.00 | 118.56 ± 1.96 | 99.63 | 1.65 | 118.39 ± 0.96 | 99.49 | 0.81 | |
| Ganoderic acid A (6) & Lucidenic acid A (7) | 30.75 | 30.28 ± 1.11 | 98.47 | 3.67 | 30.10 ± 0.59 | 97.89 | 1.96 |
| 61.50 | 60.00 ± 1.05 | 97.56 | 1.75 | 59.88 ± 0.88 | 97.37 | 1.47 | |
| 123.00 | 122.12 ± 1.27 | 99.28 | 1.04 | 121.16 ± 2.05 | 98.50 | 1.69 | |
| Ganoderic acid E (8) | 29.75 | 29.59 ± 0.59 | 99.46 | 1.99 | 29.03 ± 0.76 | 97.58 | 2.62 |
| 59.50 | 59.27 ± 1.12 | 99.61 | 1.89 | 58.36 ± 0.99 | 98.08 | 1.70 | |
| 119.00 | 118.33 ± 0.95 | 99.44 | 0.80 | 118.13±1.13 | 99.27 | 0.96 | |
| Methyl lucidenate E2 (9) | 28.75 | 27.94 ± 0.84 | 97.18 | 3.01 | 29.13 ± 0.89 | 101.32 | 3.06 |
| 57.50 | 58.24 ± 1.10 | 101.29 | 1.89 | 56.02 ± 0.93 | 97.43 | 1.66 | |
| 115.00 | 116.05 ± 1.27 | 100.91 | 1.09 | 114.12 ± 2.11 | 99.23 | 1.85 | |
| Methyl lucidenate A (10) | 31.75 | 29.62 ± 0.66 | 93.29 | 2.23 | 30.59 ± 0.73 | 96.35 | 2.39 |
| 63.50 | 62.21 ± 0.98 | 97.97 | 1.58 | 62.58 ± 1.06 | 98.55 | 1.69 | |
| 127.00 | 125.76 ± 0.74 | 99.02 | 0.59 | 126.29 ± 1.94 | 99.44 | 1.54 | |
| Butyl lucidenate E2 (11) and Butyl ganoderate A (12) | 33.75 | 29.45 ± 0.58 | 87.26 | 1.97 | 32.19 ± 0.82 | 95.38 | 2.55 |
| 67.50 | 66.54 ± 1.20 | 98.58 | 1.80 | 66.83 ± 1.02 | 99.01 | 1.53 | |
| 135.00 | 133.50 ± 1.31 | 98.89 | 0.98 | 134.20 ± 2.07 | 99.41 | 1.54 | |
| Lucidadiol (13) | 32.50 | 30.08 ± 0.69 | 92.55 | 2.29 | 32.19 ± 0.83 | 99.05 | 2.58 |
| 65.00 | 63.89 ± 1.01 | 98.29 | 1.58 | 63.97 ± 1.32 | 98.42 | 2.06 | |
| 130.00 | 128.95 ± 1.17 | 99.19 | 0.91 | 128.04 ± 1.95 | 98.49 | 1.52 | |
| Ganodemanontriol (14) | 30.75 | 29.85 ± 0.58 | 97.07 | 1.94 | 29.83 ± 0.59 | 97.01 | 1.98 |
| 61.50 | 61.59 ± 0.94 | 100.15 | 1.53 | 60.82 ± 0.83 | 98.89 | 1.36 | |
| 123.00 | 122.11 ± 1.27 | 99.28 | 1.04 | 122.39 ± 1.96 | 99.50 | 1.60 | |
| Ganoderiol F (15) | 29.25 | 29.78 ± 0.55 | 101.81 | 1.85 | 29.89 ± 0.55 | 102.19 | 1.84 |
| 58.50 | 58.02 ± 1.01 | 99.18 | 1.74 | 58.01 ± 1.03 | 99.16 | 1.78 | |
| 117.00 | 115.38 ± 1.78 | 98.62 | 1.54 | 116.12 ± 2.11 | 99.25 | 1.82 | |
| Ganodermadiol (16) | 30.25 | 30.05 ± 0.73 | 99.34 | 2.43 | 30.11 ± 0.49 | 99.54 | 1.63 |
| 60.50 | 60.02 ± 0.79 | 99.21 | 1.32 | 60.12 ± 1.37 | 99.37 | 2.28 | |
| 121.00 | 120.11 ± 1.02 | 99.26 | 0.85 | 120.37 ± 2.05 | 99.48 | 1.70 | |
| Ergosterol (17) | 29.75 | 30.67 ± 0.63 | 103.09 | 2.05 | 30.17 ± 0.73 | 101.41 | 2.42 |
| 59.50 | 58.03 ± 0.92 | 97.53 | 1.59 | 59.20 ± 1.52 | 99.50 | 2.57 | |
| 119.00 | 119.97 ± 1.12 | 100.82 | 0.93 | 118.79 ± 1.99 | 99.82 | 1.68 | |
| Compounds | Content (µg/g) a | |||||
|---|---|---|---|---|---|---|
| VN1 | VN12 | VN13 | VN16 | VN18 | VN21 | |
| Acid | ||||||
| Lucidenic acid N (4) | 388.16 ±5.10 | 444.58 ± 3.84 | 845.46 ± 9.89 | 420.71 ± 2.40 | 257.80 ± 3.88 | 884.05 ± 7.45 |
| Lucidenic acid E2 (5) | 776.90 ± 9.83 | 1766.75 ± 24.01 | 319.47 ± 6.93 | 1151.08 ± 7.64 | 430.79 ± 7.04 | 1695.01 ± 16.32 |
| Ganoderic acid A (6) & Lucidenic acid A (7) | 228.88 ± 3.12 | 401.93 ± 2.37 | 349.93 ± 5.05 | 140.97 ± 1.71 | 891.05 ± 7.68 | 509.76 ± 7.05 |
| Ganoderic acid E (8) | 878.27 ± 7.75 | 1528.73 ± 12.88 | 574.54 ± 4.71 | 797.83 ± 18.76 | 2100.20 ± 13.11 b | 1614.25 ± 9.27 |
| Methyl lucidenate E2 (9) | − | − | − | − | − | − |
| Methyl lucidenate A (10) | 46.62 ± 1.47 | − | − | − | − | − |
| Butyl lucidenate E2 (11) & Butyl ganoderate A (12) | − | − | − | − | − | − |
| Total acid | 2318.82 ± 6.24 | 4141.98 ± 22.15 | 2089.40 ± 24.29 | 2510.58 ± 29.77 | 3679.85 ± 21.66 | 4703.07 ± 38.72 |
| Alcohol | ||||||
| Lucidadiol (13) | 144.44 ± 2.32 | 212.81 ± 1.35 | 111.31 ± 1.00 | 128.84 ± 0.54 | 243.95 ± 6.43 | 219.25 ± 2.00 |
| Ganodemanontriol (14) | 168.35 ± 2.53 | 364.27 ± 3.33 | 232.15 ± 1.82 | 129.31 ± 0.95 | 394.10 ± 5.78 | 291.04 ± 2.44 |
| Ganoderiol F (15) | 697.42 ± 13.75 | 643.68 ± 11.49 | 795.69 ± 5.07 | 563.94 ± 2.86 | 1635.06 ± 13.63 | 689.01 ± 2.02 |
| Ganodermadiol (16) | 218.99 ± 4.14 | 159.87 ± 0.95 | 91.38 ± 1.03 | 95.33 ± 0.60 | 225.56 ± 0.49 | 348.52 ± 4.90 |
| Total alcol | 1229.20 ± 21.16 | 1380.63 ± 14.30 | 1230.54 ± 6.38 | 917.41 ± 3.65 | 2498.68 ± 7.41 | 1547.83 ± 9.55 |
| Other | ||||||
| Ergosterol (17) | 205.82 ± 1.72 | 700.74 ± 4.76 | 222.27 ± 4.93 | 325.85 ± 1.87 | 862.09 ± 12.71 | 160.74 ± 4.56 |
| Compounds | Content (µg/g) a | |||
|---|---|---|---|---|
| GL1 | GL2 | GL3 | VN10 | |
| Acid | ||||
| Lucidenic acid N (4) | 139.08 ± 1.82 | − | 79.58 ± 1.47 | 52.53 ± 1.06 |
| Lucidenic acid E2 (5) | 481.31 ± 4.07 | 420.91 ± 2.78 | 357.89 ± 2.90 | 258.06 ± 1.31 |
| Ganoderic acid A (6) & Lucidenic acid A (7) | 83.35 ± 2.40 | 232.55 ± 5.66 | 70.17 ± 0.66 | 76.19 ± 0.18 |
| Ganoderic acid E (8) | 730.00 ± 4.27 | 85.54 ± 0.89 | 207.77 ± 1.79 | 773.92 ± 4.75 |
| Methyl lucidenate E2 (9) | 286.94 ± 2.33 | 446.45 ± 8.63 | 288.41 ± 1.73 | − |
| Methyl lucidenate A (10) | − | − | − | − |
| Butyl lucidenate E2 (11) & Butyl ganoderate A (12) | − | − | − | − |
| Total acid | 1720.69 ± 9.23 | 1185.46 ± 3.01 | 1003.83 ± 5.02 | 1160.36 ± 7.87 |
| Alcohol | ||||
| Lucidadiol (13) | 69.15 ± 1.40 | 49.43 ± 0.69 | 109.00 ± 1.00 | 50.47 ± 1.59 |
| Ganodemanontriol (14) | 107.04 ± 3.32 | 208.34 ± 2.62 | 50.85 ± 0.80 | |
| Ganoderiol F (15) | 86.16 ± 1.24 | 65.03 ± 0.71 | 153.75 ± 0.92 | 226.71 ± 1.29 |
| Ganodermadiol (16) | 59.59 ± 0.60 | 78.23 ± 0.64 | 37.07 ± 0.41 | |
| Total alcol | 155.31 ± 1.38 | 281.10 ± 4.06 | 549.32 ± 3.44 | 365.10 ± 0.93 |
| Other | ||||
| Ergosterol (17) | 135.14 ± 1.52 | 194.68 ± 3.74 | 795.96 ± 5.65 | 647.79 ± 10.62 |
| Compounds | Content (µg/g) a | ||||
|---|---|---|---|---|---|
| TGLs | TGap | TGau | TGc | TGs | |
| Acid | |||||
| Lucidenic acid N (4) | 144.18 ± 1.15 | − | 63.13 ± 1.45 | 207.73 ± 2.05 | 57.50 ± 0.65 |
| Lucidenic acid E2 (5) | 413.76 ± 2.04 | − | 121.65 ± 4.50 | 201.92 ± 2.45 | − |
| Ganoderic acid A (6) & Lucidenic acid A (7) | 89.18 ± 0.97 | − | 78.09 ± 2.97 | 118.24 ± 3.09 | − |
| Ganoderic acid E (8) | 475.23 ± 3.28 | 114.89 ± 1.89 | 1213.14 ± 15.26 | 337.62 ± 4.91 | 51.10 ± 1.30 |
| Methyl lucidenate E2 (9) | − | 2499.52 ± 17.65 | 18,247.10 ± 48.40 b | 1023.84 ± 28.71 | − |
| Methyl lucidenate A (10) | − | − | 144.89 ± 3.34 | − | − |
| Butyl lucidenate E2 (11) & Butyl ganoderate A (12) | − | − | 65.65 ± 0.65 | − | − |
| Total acid | 1119.26 ± 4.48 | 2613.80 ± 17.08 | 19,999.28 ± 41.66 | 1889.35 ± 20.91 | 108.60 ± 1.07 |
| Alcohol | |||||
| Lucidadiol (13) | 54.04 ± 1.32 | 70.36 ± 0.06 | 98.44 ± 1.19 | 46.08 ± 0.78 | − |
| Ganodemanontriol (14) | − | 72.99 ± 1.88 | − | ||
| Ganoderiol F (15) | 84.61 ± 0.85 | − | 126.42 ± 1.32 | 272.73 ± 3.49 | 56.58 ± 0.70 |
| Ganodermadiol (16) | − | − | 47.55 ± 0.71 | ||
| Total alcol | 138.65 ± 1.48 | 70.36 ± 0.06 | 224.86 ± 1.23 | 439.35 ± 5.22 | 56.58 ± 0.70 |
| Other | |||||
| Ergosterol (17) | 116.32 ± 1.59 | 112.71 ± 0.52 | 148.30 ± 2.63 | 221.45 ± 2.49 | 158.35 ± 1.92 |

2.4. Discussion
3. Experimental Section
3.1. Ganoderma Species
3.2. Standard Compounds
3.3. Chemicals and Reagents
3.4. Reference Compound Preparation
3.5. HPLC
3.6. Method Validation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, S.C.; Birmingham, J.M. Medicinal benefits of the mushroom Ganoderma. Adv. Appl. Microbiol. 1992, 37, 101–134. [Google Scholar] [PubMed]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotech. Ann. Rev. 2007, 13, 265–301. [Google Scholar]
- Kim, H.W.; Kim, B.K. Biomedicinal triterpenoids of Ganoderma lucidum (Curt.: Fr.) P. Karst (aphyllophoromycetidae). Intern. J. Med. Mushrooms 1999, 1, 121–138. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Kodora, Y.; Shimizu, M.; Sonoda, Y.; Sato, Y. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem. Pharm. Bull. 1990, 38, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Akiba, M.; Imaizumi, T.; Kinoshita, K.; Takahashi, K.; Suzuki, A.; Yano, S.; Horie, S.; Watanabe, K. Antinociceptive constituents of Auricularia polytricha. Planta Med. 2002, 68, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Oh, J.; Khoi, N.M.; Dao, T.T.; Dung, L.V.; Do, T.N.Q.; Lee, S.M.; Jang, T.S.; Jeong, G.S.; Na, M. In vitro and in vivo hepatoprotective effect of ganodermanontriol against t-BHP-induced oxidative stress. J. Ethnopharmacol. 2013, 150, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Zhang, Q.; Yamamoto, T.; Tayama, M.; Hattori, M.; Tanaka, K.; Kuraishi, Y. Inhibitory effects of the methanol extract of Ganoderma lucidum on mosquito allergy-induced itch-associated responses in mice. J. Pharmacol. Sci. 2010, 114, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.D. The pharmacological potential of mushrooms. eCAM 2005, 2, 285–299. [Google Scholar] [PubMed]
- Ma, J.-Q.; Liu, C.-M.; Qin, Z.-H.; Jiang, J.-H.; Sun, Y.-Z. Ganoderma applanatum terpenes protect mouse liver against benzo(α)pyren-induced oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2011, 31, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-J.; Nakamura, N.; Min, B.-S.; Hirakawa, A.; Zuo, F.; Hattori, M. Quantitative determination of bitter principles in specimens of Ganoderma lucidum using high-performance liquid chromatography and its application to the evaluation of ganoderma products. Chem. Pharm. Bull. 2004, 52, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Keypour, S.; Rafati, H.; Riahi, H.; Mirzajani, F.; Fata Moradali, M. Qualitative analysis of ganoderic acids in Ganoderma lucidum from iran and china by rp-hplc and electrospray ionisation-mass spectrometry (ESI-MS). Food Chem. 2010, 119, 1704–1708. [Google Scholar] [CrossRef]
- Ruan, W.; Popovich, D.G. Ganoderma lucidum triterpenoid extract induces apoptosis in human colon carcinoma cells (Caco-2). Biomed. Prev. Nutr. 2012, 2, 203–209. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Xie, M.-Y.; Nie, S.-P.; Liu, W.; Gong, X.-F.; Wang, Y.-X. Development of a chromatographic fingerprint for the chloroform extracts of Ganoderma lucidum by hplc and LC–MS. J. Pharm. Biomed. Anal. 2008, 47, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Sabulal, B.; George, V.; Smina, T.P.; Janardhanan, K.K. Antioxidative and anti-inflammatory activities of the chloroform extract of Ganoderma lucidum found in south india. Sci. Pharm. 2009, 77, 111–121. [Google Scholar] [CrossRef]
- Salteralli, R.; Ceccaroli, P.; Lotti, M.; Zambonelli, A.; Buffalini, M.; Casadei, L. Biochemical characterization and antioxidant activity of mycelium of Ganoderma lucidum from central italy. Food Chem. 2009, 116, 143–151. [Google Scholar] [CrossRef]
- Nguyen, P.D.N.; Do, H.T.; Le, B.D. Characteristics of ecological factors and their distribution of Ganodermataceae Donk. in highlands of vietnam. J. Biol. 2013, 35, 198–205. [Google Scholar]
- Dam, N.; Nguyen, G.C.; Nguyen, B.; Trinh, T.K. Ganoderma species in vietnam. Hanoi-J. Med. Mat. 1997, 2, 10–13. [Google Scholar]
- Tung, N.T.; Cuong, T.D.; Hung, T.M.; Lee, J.H.; Woo, M.H.; Choi, J.S.; Kim, J.; Ryu, S.H.; Min, B.S. Inhibitory effect on no production of triterpenes from the fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2013, 23, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Thu, N.T.; Khoi, N.M. Derivatives of nucleic and benzoic acids isolated from Ganoderma lucidum collected in quangnam-danang, vietnam. Hanoi-J. Med. Mat. 2014, 19, 216–219. [Google Scholar]
- Yuan, J.-P.; Wang, J.-H.; Liu, X.; Kuang, H.-C.; Huang, X.-N. Determination of ergosterol in ganoderma spore lipid from the germinating spores of Ganoderma lucidum by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 6172–6176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Yang, M.; Guan, S.H.; Liu, R.X.; Xia, J.M.; Bi, K.S.; Guo, D.A. Quantitative determination of six major triterpenoids in Ganoderma lucidum and related species by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2006, 41, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Seo, J.; Kim, J.; Kim, H.; Youn, U.; Lee, J.; Jung, H.; Na, M.; Hattori, M.; Min, B.; et al. Lanostane triterpenes from the fruiting bodies of Ganoderma lucidum and their inhibitory effects on adipocyte differentiation in 3T3-L1 cells. J. Nat. Prod. 2010, 73, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Thuy, P.T.; Khoi, N.M. Ergostane and lanostane steroids from fruiting body of Ganoderma lucidum collected in quangnam, danang. Hanoi-J. Med. Mat. 2013, 18, 389–394. [Google Scholar]
- Zhao, J.; Zhang, X.Q.; Li, S.P.; Yang, F.Q.; Wang, Y.T.; Ye, W.C. Quality evaluation of Ganoderma through simultaneous determination of nine triterpenes and sterols using pressurized liquid extraction and high performance liquid chromatography. J. Sep. Sci. 2006, 29, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Qin, J.-Z.; Chen, P.; Chen, X.; Zhang, Y.-Z.; Zhao, S.-J. Quality difference study of six varieties of Ganoderma lucidum with different origins. Front. Pharmacol. 2012, 3, 57. [Google Scholar] [PubMed]
- Gao, J.L.; Leung, K.S.Y.; Wang, Y.T.; Lai, C.M.; Li, S.P.; Hu, L.F.; Lu, G.H.; Jiang, Z.H.; Yu, Z.L. Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. By HPLC-DAD-MS. J. Pharm. Biomed. Anal. 2007, 44, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Qiu, F.; Di, X. Sensitive and selective liquid chromatography-tandem mass spectrometry method for the determination of five ganoderic acids in Ganoderma lucidum and its related species. J. Pharm. Biomed. Anal. 2011, 54, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, D.T.; Loan, L.T.; Hung, T.M.; Han, L.V.N.; Khoi, N.M.; Dung, L.V.; Min, B.S.; Nguyen, N.P.D. An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma lucidum and Its Five Related Species Originating from Vietnam. Molecules 2015, 20, 1059-1077. https://doi.org/10.3390/molecules20011059
Ha DT, Loan LT, Hung TM, Han LVN, Khoi NM, Dung LV, Min BS, Nguyen NPD. An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma lucidum and Its Five Related Species Originating from Vietnam. Molecules. 2015; 20(1):1059-1077. https://doi.org/10.3390/molecules20011059
Chicago/Turabian StyleHa, Do Thi, Le Thi Loan, Tran Manh Hung, Le Vu Ngoc Han, Nguyen Minh Khoi, Le Viet Dung, Byung Sun Min, and Nguyen Phuong Dai Nguyen. 2015. "An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma lucidum and Its Five Related Species Originating from Vietnam" Molecules 20, no. 1: 1059-1077. https://doi.org/10.3390/molecules20011059






