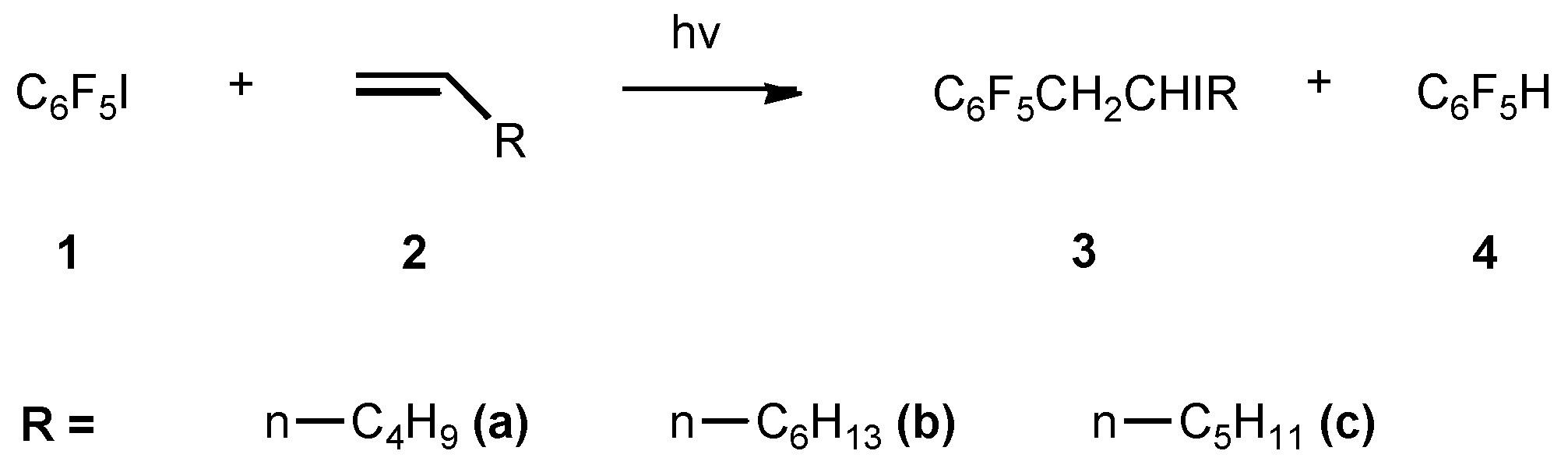M.P.s are uncorrected. IR spectra were obtained on a Schimadzu-440 instrument in potassium bromide pellets for all solid samples and in films for all liquid samples. 1H NMR spectra were recorded on a Jeol FX-90Q instrument using tetramethylsilane as an internal standard. 19F NMR spectra were recorded on a Varian EM-360 instrument at 56.4 MHz using trifluoroacetic acid as an external standard with chemical shifts in ppm positive upfield. Mass spectra was obtained on a Finnigan-4021 instrument. Silica gel (10-40m) was used for column chromatography.
Typical procedure of the reaction of pentafluoroiodoben-zene (1) with alkene (2) and (5)
Under a N2 atmosphere, a stirred mixture of 1 (2.94g, 10mmol) and 1-hexene (8.4g, 100mmol) in a quartz flask, connected to a condenser, was exposed to a high pressure mercury lamp (300W) at a distance of 8cm for 12h. 19F NMR, showed that 10% of pentafluorobenzene (4) was formed. The mixture was concentrated under reduced pressure and was subjected to column chromatography, using light petroleumether as eluent to give the product 3a (0.68g, 30%).
2-Iodo-1-pentafluorophenyl hexane (3a)
νmax/cm-1: 2890, 1530, 1510, 1130, 950-1020; δH(CDCl3): 4.20 (m, 1H), 3.30 (d, 2H, J=6), 1.63 (m, 2H), 1.33 (m, 4H) and 0.88 (s, 3H)ppm; δF(CDCl3): 64.3 (m, 2F), 78.9 (t, 1F), 84.3 (m, 2F) ppm; m/z: 251 (M+-I, 31%), 181(C6F5CH2+, 100%), 127 (2.7%), 57 (30%); Found: C, 38.18; H, 3.36; F, 25.25%; Calc. for C12H12F5I: C, 38.10; H, 3.17; F, 25.13%.
2-Iodo-1-pentafluorophenyl octane (3b)
νmax/cm-1: 2890, 1530, 1510, 1120; δH(CDCl3): 4.27 (m, 1H), 3.33 (d, 2H, J=6), 0.90-2.0 (m, 13H) ppm; δF(CDCl3): 66.0 (m, 2F), 80.0 (t, 1F), 86.2 (m, 2F) ppm; m/z: 279 (M+-I, 12.4%), 278 (M+-HI, 46.1%), 194 (M+-I-C6H13, 56.8%), 181 (C6F5CH2+, 100%), 167 (5.4%); Found: C, 41.38; H, 3.94; F, 23.40%; Calc. for C12H12F5I: C, 41.39; H, 3.97; F, 23.38%.
2-Iodo-1-pentafluorophenyl heptane (3c)
νmax/cm-1: 2890, 1530, 1500, 1120; δH(CDCl3): 4.27 (m, 1H), 3.33 (d, 2H, J=6), 0.90-2.20 (m, 11H) ppm; δF(CDCl3): 65.0 (m, 2F), 79.0 (t, 1F), 85.2 (m, 2F) ppm; m/z: 265 (M+-I, 2.4%), 264 (M+-HI, 16.1%), 194 (M+-I-C5H11, 100%), 181 (C6F5CH2+, 35.2%), 167 (7.4%); HRMS (for C13F5H13, M+-HI), Calc: 264.0937, Found: 264.0922.
C6F5CH2CH(I)CH2OC2H5 (6a)
νmax/cm-1: 2900, 1740, 1500, 1220; δH(CDCl3): 3.10-4.24 (m, 7H), 1.80 (t, 3H)ppm; δF(CDCl3): 65.7 (m, 2F), 79.5 (t, 1F), 85.3 (m, 2F) ppm; m/z: 353 (M+-I, 34.0%), 224 (M+-IC2H5, 29%), 181 (C6F5CH2+, 100%), Found: C, 34.70; H, 2.56; F, 24.50%; Calc. for C11H10F5IO: C, 34.74; H, 2.62; F, 25.00%.
C6F5CH2CHICH2OAc (6b)
νmax/cm-1: 2900, 1740, 1500, 1220; δH(CDCl3): 4.37 (m, 3H), 3.37 (m, 2H), 2.13 (s, 3H)ppm; δF(CDCl3): 66.7 (m, 2F), 80.3 (t, 1F), 86.7 (m, 2F) ppm; m/z: 395 (M++1, 0.77%), 335 (M+-AcO, 29%), 267 (M+-I, 100%), 207 (M+-AcO-HI, 67%), 181 (C6F5CH2+, 49%), 43 (AcO+, 84%), Found: C, 33.56; H, 1.99; F, 24.52%; Calc. for C11H8F5IO2: C, 33.50; H, 2.03; F, 24.52%.
Typical procedure of the reaction of pentafluoroiodoben-zene (1) with aryl allyl ether 7
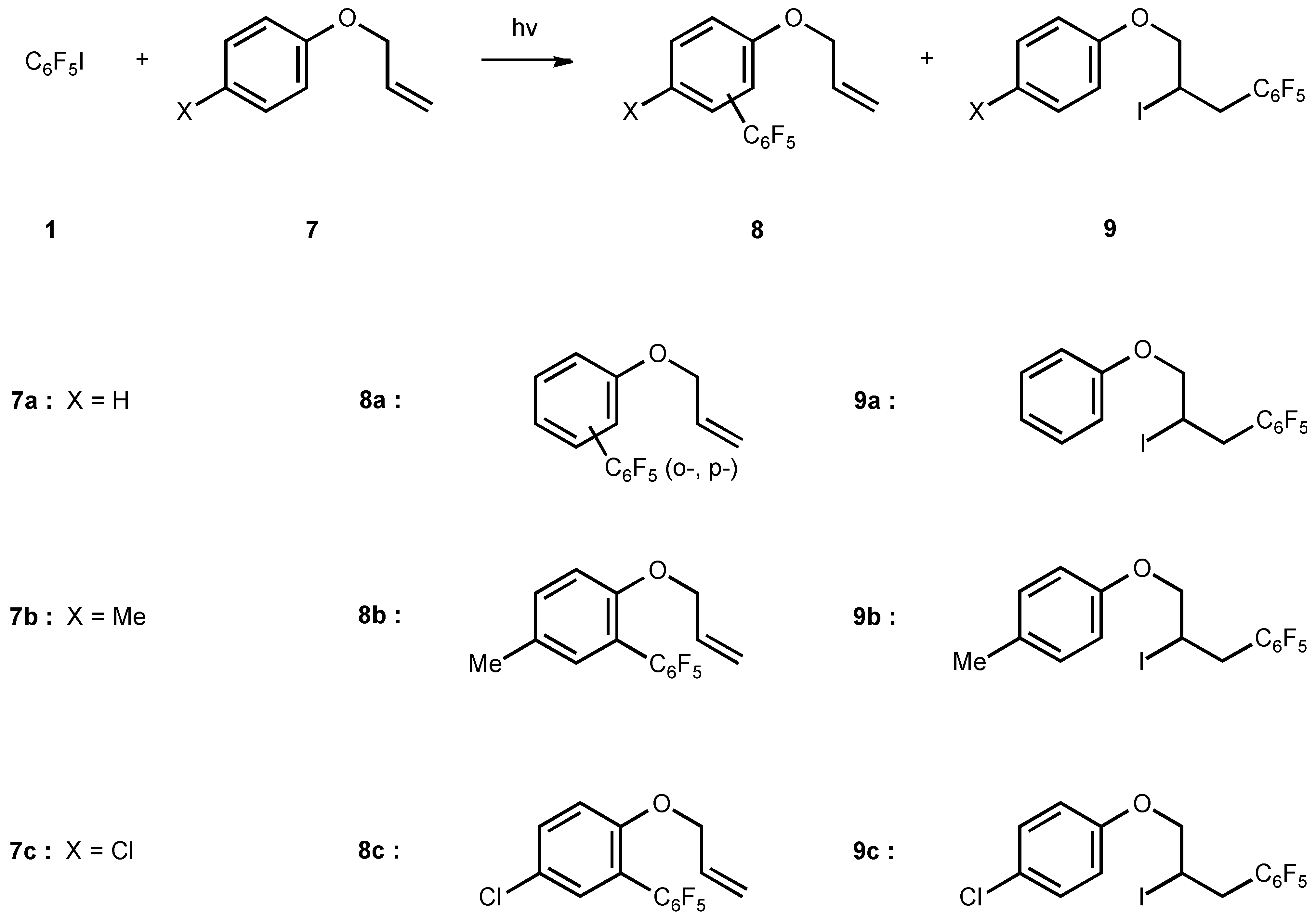
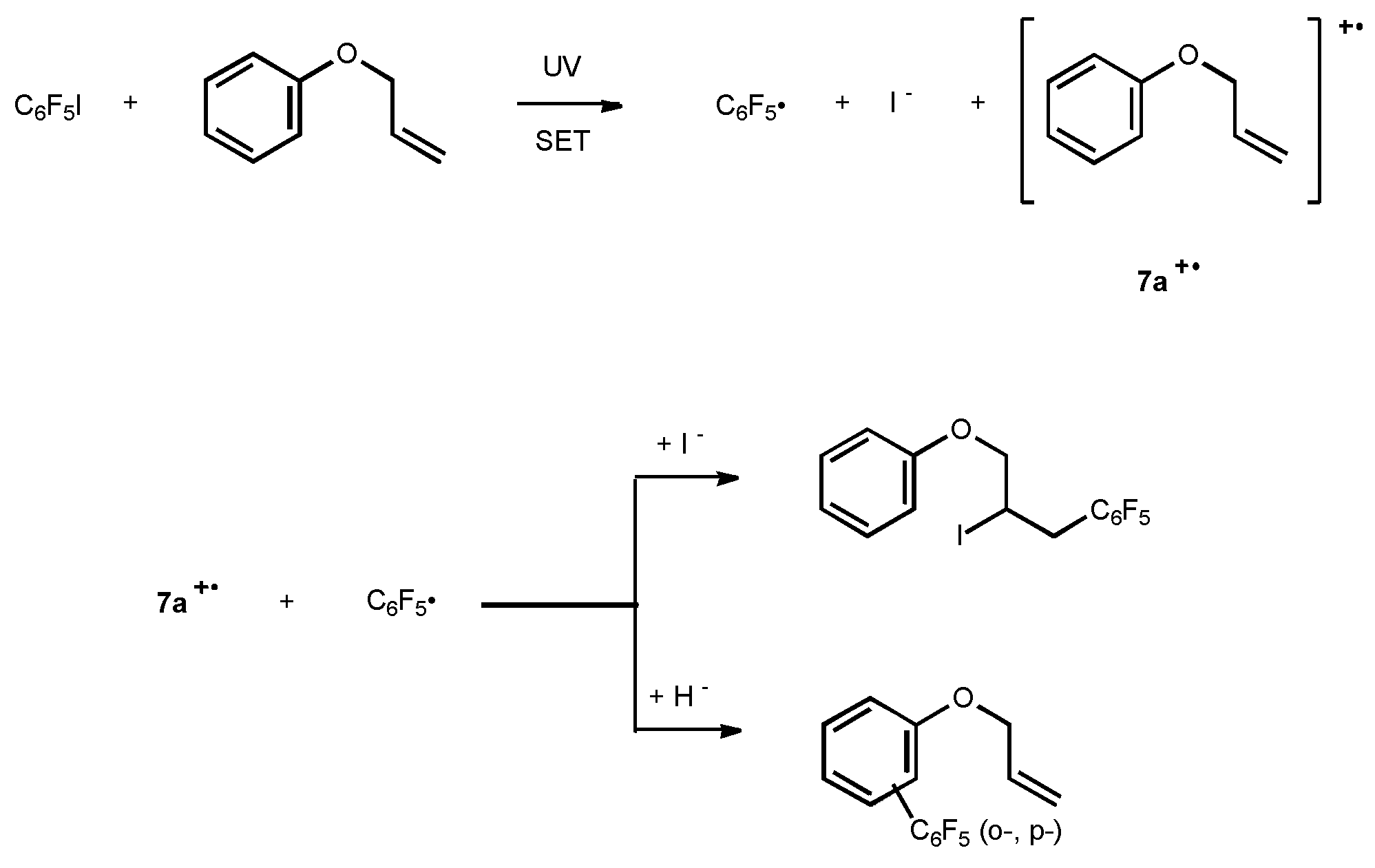
Under a N2 atmosphere, the mixture of 1 (2.94g, 10mmol), 7a (10g, 0.1mol) and CH3CN(20ml) was irradiated for 12h as above. After work-up, the excess of 7a was then distilled off in vacuum, the oily residue was subject to column chromatography using petroleum ether as eluent to give product 8a (0.6g, 36%) and 9a (0.4g, 24%).
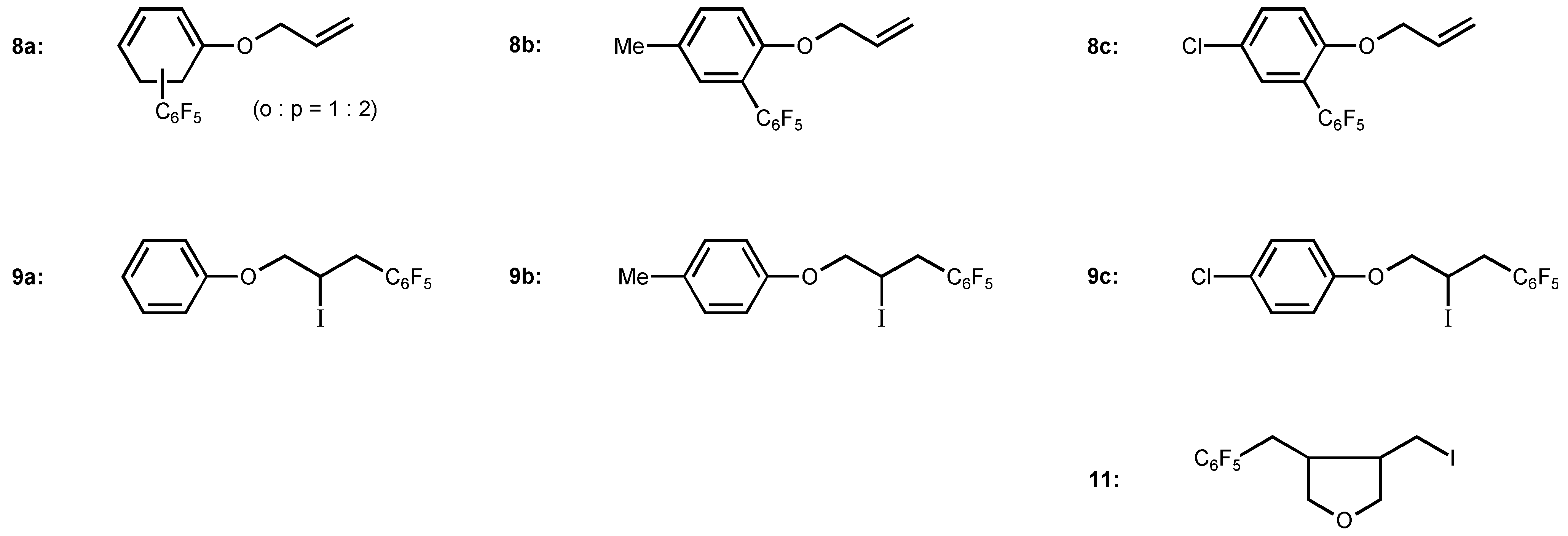
8a (Formula see Scheme 7)
νmax/cm-1: 1640, 1520, 1480, 1280, 1060, 860, 820; δH(CDCl3): 7.0 (m, 4H), 5.9 (m, 1H), 5.25 (m, 2H), 4.5 (m, 2H) ppm; δF(CDCl3): 63.7 (m, 2F, p-), 65.7 (m, 2F, o-), 78.7 (t, 1F), 85.0 (m, 2F) ppm; m/z: 300 (M+, 100%), 260 (M+-C3H4, 18%), 231 (M+-CO-C3H5, 46%), 205 (M+-C3H5-C2H2, 24%), 181 (C6F5CH2+, 70%), HRMS (for C15H9F5O): Calc: 300.2220, Found: 300.1599.
9a (Formula see Scheme 7)
νmax/cm-1: 1580, 1500, 1230, 980, 750, 680; δH(CDCl3): 7.10 (m, 5H), 4.42 (m, 1H), 4.20 (m, 2H), 3.40 (m, 2H) ppm; δF(CDCl3): 66.3 (m, 2F), 78.7 (t, 1F), 85.0 (m, 2F) ppm; m/z: 427 (M+, 18.0%), 300 (M+-I, 100%), 181 (C6F5CH2+, 70%), 94 (C6H5OH+, 25.0%), Found: C, 42.32; H, 2.39; Calc. for C15H10F5IO: C, 42.08; H, 2.35.
8b (Formula see Scheme 7)
νmax/cm-1: 1640, 1600, 1580, 1500, 1280, 1220, 1120, 980, 910, 800; δH(CDCl3): 6.95 (m, 3H), 5.9 (m, 1H), 5.20 (m, 2H), 4.40 (d, 2H), 2.21 (s, 3H) ppm; δF(CDCl3): 63.0 (m, 2F), 79.0 (t, 1F), 85.3 (m, 2F) ppm; m/z: 314 (M+, 100%), 299 (M+-CH3, 36%), 273 (M+-C3H5, 12%), 245 (M+-COC3H5, 16%). HRMS (for C16H11F5O): Calc: 314.2543, Found: 314.0768.
9b (Formula see Scheme 7)
M.p.: 71-73°C. νmax/cm-1: 2860, 1520, 1500, 1370, 1220, 1120, 980, 960; δH(CDCl3): 6.90 (AA’BB’, 4H), 4.4 (m, 1H), 4.20 (s, 2H), 3.40 (m, 2H), 2.23 (s, 3H) ppm; δF(CDCl3): 65.0 (m, 2F), 78.0 (t, 1F), 85.3 (m, 2F) ppm; m/z: 442 (M+, 26%), 315 (M+-CH3, 36%), 181 (C6F5CH2+, 82%), 108 (p-MeC6H4OH+, 100%). Found: C, 43.32; H, 2.39; Calc. for C16H12F5IO: C, 43.44; H, 2.71.
8c (Formula see Scheme 7)
νmax/cm-1: 2960, 1640, 1590, 1580, 1520, 1480 1280, 1220, 1010, 980; δH(CDCl3): 7.17-6.83 (m, 3H), 5.90 (m, 1H), 5.30 (m, 2H), 4.62 (s, 2H) ppm; δF(CDCl3): 63.0 (m, 2F), 78.3 (t, 1F), 86.0 (m, 2F) ppm; m/z: 334 (M+, 100%), 293 (M+-C3H5, 32%), 265 (M+-CO-C3H5, 45%). HRMS (for C15H8ClF5O): Calc: 334.6640, Found: 334.6216.
9c (Formula see Scheme 7)
M.p.: 59-60°C. νmax/cm-1: 1600, 1540, 1510, 1498, 1240, 1000; δH(CDCl3): 7.27 (AA’BB’, 4H), 4.40 (m, 2H), 4.25(d, 1H, J=4Hz), 3.43 (m, 2H) ppm; δF(CDCl3): 65.0 (m, 2F), 78.3 (t, 1F), 84.7 (m, 2F) ppm; m/z: 462 (M+, 50%), 335 (M+-I, 25%), 181 (C6F5CH2+, 100%), 128 (p-ClC6H4OH+, 25%), Found: C, 39.25; H, 1.81; F, 20.40%; Calc. for C15H9ClF5IO: C, 38.96; H, 1.94; F, 20.56%.
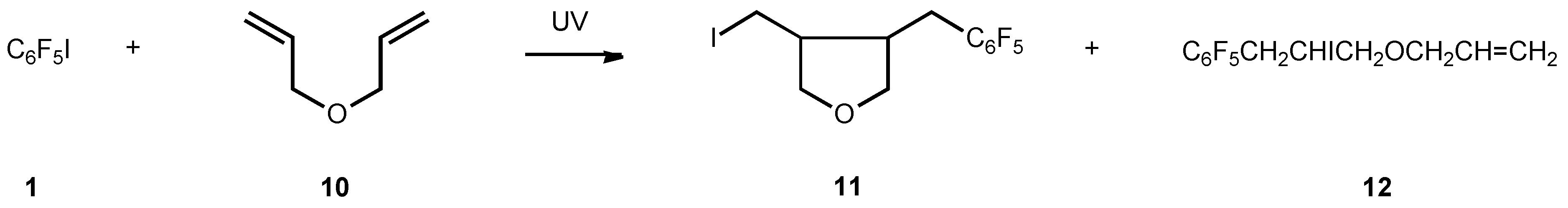
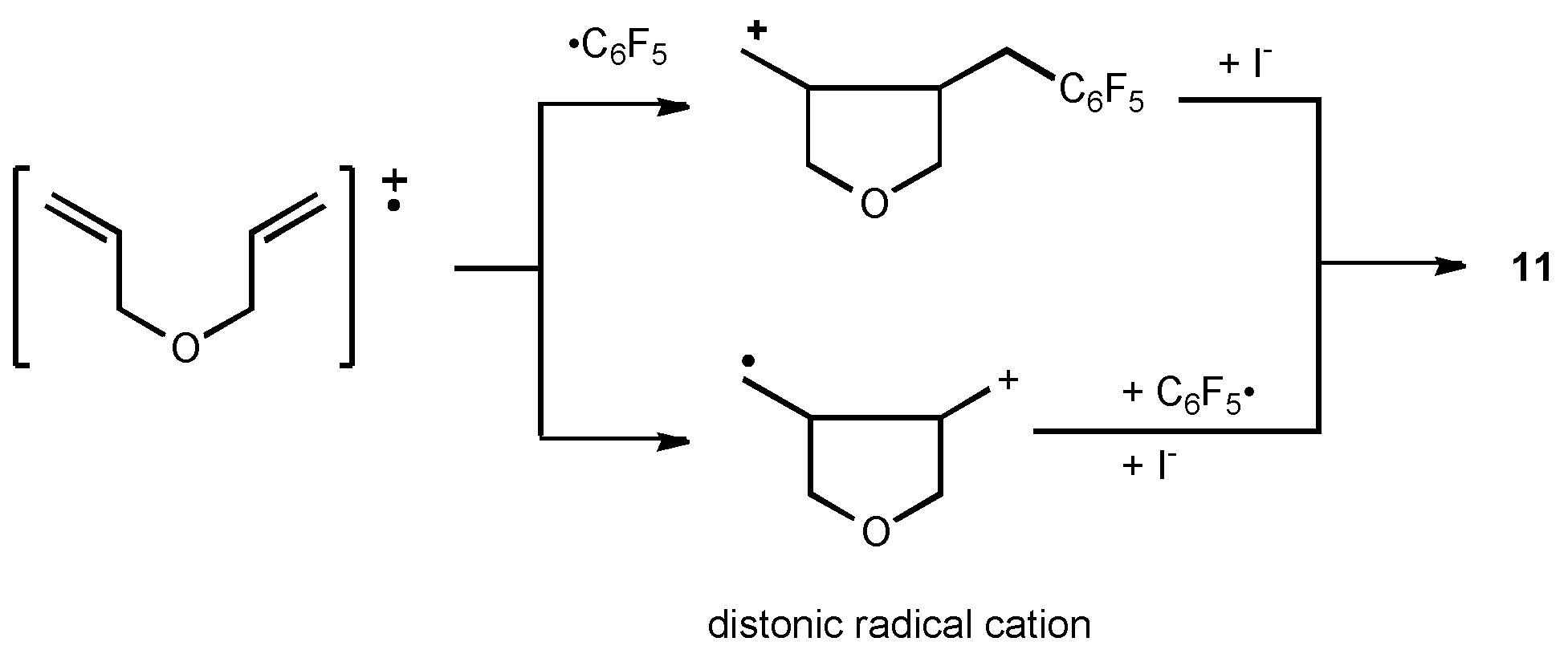
11 (Formula see Scheme 7)
M.p.: 42-44°C. νmax/cm-1: 1520, 1500, 1120, 980; δH(CDCl3): 4.00 (d, 2H, J=6Hz), 3.70 (m, 4H), 3.26 (m, 2H), 2.80 (m, 2H) ppm; δF(CDCl3): 66.0 (m, 2F), 79.7 (t, 1F), 85.3 (m, 2F) ppm; m/z: 392 (M+, 19%), 265 (M+-I, 60%), 235 (M+-I-CH2O, 22%), 181 (C6F5CH2+, 100%), Found: C, 37.51; H, 2.57; F, 24.45%; Calc. for C12H10F5IO: C, 37.42; H, 2.55; F, 24.23%.
C6F5CH2CHIOCH2CH=CH2 (12)
νmax/cm-1: 2800, 1650,1500, 1110, 980; δH(CDCl3): 5.80 (m, 1H), 5.23 (m, 2H), 4.32 (m, 1H), 4.00 (d, 2H, J=4Hz) ppm; δF(CDCl3): 65.0 (m, 2F), 79.0 (t, 1F), 85.0 (m, 2F) ppm; m/z: 392 (M+, 0.68%), 335 (M+-C3H5O, 63%), 181 (C6F5CH2+, 100%), 127 (I+, 4.5%), Found: C, 37.74; H, 2.55; F, 24.23%; Calc. for C12H10F5IO: C, 37.42; H, 2.55; F, 24.23%.