1. Introduction
Cranberry (
Vaccinium macrocarpon, Ericaceae), a native plant of North America, is among the top selling dietary supplements around the world. The juice as well as dietary supplements derived from this berry exert various beneficial effects on human health, including prevention and treatment of urinary tract infections, anti-cancer and antioxidant activities [
1,
2]. However, the positive effect of cranberry juice in the prevention of urinary tract infections has recently been disputed [
3,
4]. The biological activities of cranberry can be attributed to a diverse group of phytochemicals, including phenylpropanoids (such as flavonoids and resveratrol), phenolic acids and isoprenoids such as lutein and ursolic acid [
5,
6]. Cranberries also represent a very good source of vitamin C [
7]. Flavonoid constituents found in cranberry fruit belong primarily to three classes: proanthocyanidins, anthocyanins and flavonols [
8]. Proanthocyanidins (also known as polyflavan-3-ols) found in cranberry fruits are primarily dimers, trimers and larger oligomers of (‒)-epicatechin, containing two types of linkages between epicatechin units: the more common C4β → C8 (B-type) linkage and a less common A-type linkage featuring both C4β → C8 and C2β → O7 interflavanoid bonds [
9]. Among anthocyanins, glycosides of cyanidin, peonidin and petunidin prevail. The most abundant flavonol aglycone is quercetin, followed by myricetin and kaempferol. Cranberry fruits contain up to 91.5 mg of anthocyanins, 40 mg of flavonols and 180 mg of proanthocyanidins (with degrees of polymerization ≤ 10) per 100 g of ripe fruit [
5].
Consumption of cranberry juice and/or various dietary supplements containing cranberries have always been considered safe, however, several studies have suggested that cranberry juice is capable of interacting with drug-metabolizing enzymes and thus may elicit clinically relevant interactions with certain drugs. For instance, cranberry juice inhibited the activity of cytochrome P450 3A (CYP3A)
in vitro in human liver and rat intestinal microsomes [
10]. Moreover, in rats, cranberry juice was as effective as grapefruit juice in enhancing the systemic exposure of nifedipine, the calcium channel antagonist and CYP3A substrate. In both cases, the area under the curve (AUC) of nifedipine was increased by 60% compared to saline [
10]. Indeed, enteric, but not hepatic, CYP3A-mediated first-pass metabolism of midazolam was inhibited by cranberry juice in healthy volunteers [
11]. In contrast, no interaction has been found in the human study involving CYP3A substrate cyclosporine and cranberry juice [
12]. Long-term treatment with three cranberry extracts had no effect on glutathione S-transferase (GST) activity in rats [
13]. Besides, weak inhibitory activity of cranberry extract towards UDP-glucuronosyl transferase 1A9 (UGT1A9) in human liver microsomes was reported [
14]. Individual anthocyanidins were able to significantly inhibit human and rat carbonyl reductase (CBR) and UGT
in vitro [
15].
The abovementioned data show that information about the effect of cranberry extract/juice on drug-metabolizing enzymes has been inconsistent and insufficient. The inconsistence of the results from various studies is mainly due to the use of different experimental conditions. There are differences not only in the model systems, but also in the administered substances (undefined cranberry juice, defined cranberry extract, individual anthocyanidins, etc.), and dosage schemes (various doses, various treatment durations, etc.). Moreover, most of the studies have focused only on selected enzymes in one organ (mostly liver) and a comprehensive view has been lacking.
Therefore, the present in vivo study was designed to test the effect of cranberry extract (in two different dosage schemes) on the activity and expression of a panel of drug-metabolizing enzymes (a total of seven) in rats. In addition to the hepatic enzymes most studied in the literature, intestinal enzymes were also included in this project to obtain more complete information. With the aim to increase reproducibility and repeatability of our experiments, CystiCran®, a patented and standardized cranberry extract, was used in this study.
2. Results and Discussion
Cranberry standardized extract CystiCran
® (CC, Decas Botanical Synergies, Carver, MA, USA) contains 1.6 mg of anthocyanidins and 36 mg of proanthocyanidins in one tablet, mainly those comprising A-type linkages, which have been associated with preventing adhesion of uropathogenic bacteria to uroepithelial cells [
16]. In the present study, male rats were orally treated with CC to simulate consumption of cranberry juice and/or dietary supplements containing cranberry extract. Cranberry juice as well as dietary supplements are usually taken orally, therefore
p.o. administration of CC (by gastric gavage) was chosen. The two dosage schemes employed represented two possible situations: regular long-term consumption of dietary supplement as a prevention of urinary tract infection (14 days, 0.5 mg of proanthocyanidins/kg/day,
i.e., 1 tablet of CC/day in human therapy) and short-term overdose by dietary supplement (1 day, 1.5 mg of proanthocyanidins/kg/day,
i.e., 3 tablets of CC/day in human therapy). In order to obtain more comprehensive information about the impact of cranberry extract on drug-metabolizing enzymes, their activities and expressions were studied not only in liver, but also in small intestine. Moreover, anthocyanins and proanthocyanidins are polyphenols with low absorption [
17], and thus their systemic bioavailability is low, but intestinal mucosa exposure is high.
Proanthocyanidins are stable during gastric transit. While degradation of polymeric proanthocyanidins to the corresponding monomers is negligible in the gastrointestinal tract
in vivo, proanthocyanidin oligomers with a degree of polymerization lower than 5 are absorbable [
18]. Upon absorption, all dietary polyphenols in the human body are subject to substantial transformation catalyzed by drug-metabolizing enzymes [
19]. Unlike extensive phase II metabolism of absorbed monomers, which are glucuronidated, sulfated and methylated [
19], phase II metabolism of dimers appeared to be limited because glucuronidated or sulfated metabolites of dimers were not detected in biological fluids after intestinal perfusion in rats [
18]. Nevertheless, the majority of proanthocyanidins reach the colon intact and are degraded into phenylvalerolactones and phenolic acids by colon microbiota. These microbial metabolites may contribute to the health-promoting properties of proanthocyanidins
in vivo [
18].
2.1. Effect of CystiCran® on the Activities of Phase I Biotransformation Enzymes
The phase I of drug metabolism includes oxidation, reduction or hydrolytic reactions, which lead to introduction or uncovering of functional groups (e.g., –OH, –COOH, –SH, –O– or –NH
2 group) resulting in new chemical entities with different physico-chemical and biological properties. Reactions carried out by phase I enzymes usually transform active drugd into their inactive metabolite(s), but in certain cases, phase I biotransformation causes bioactivation of xenobiotics. Enzymes responsible for phase I biotransformation of drugs and other xenobiotics are abundantly present in the liver, gastrointestinal tract, lungs and kidneys. Many of them are inducible by various xenobiotics. Induction of drug metabolism may arise as a consequence of increased synthesis, decreased degradation, activation of enzymes or a combination of these three processes, although it should be emphasized that the majority of enzymes are induced at the transcriptional activation level. Thus, enzyme induction takes place only after its prolonged exposure to the xenobiotic [
20]. Cytochromes P450 (CYP), a superfamily of the most important drug-metabolizing enzymes, are involved in the metabolism of about 75% of all drugs. These enzymes utilize one molecule of oxygen and produce oxidized substrates and a molecule of water [
21].
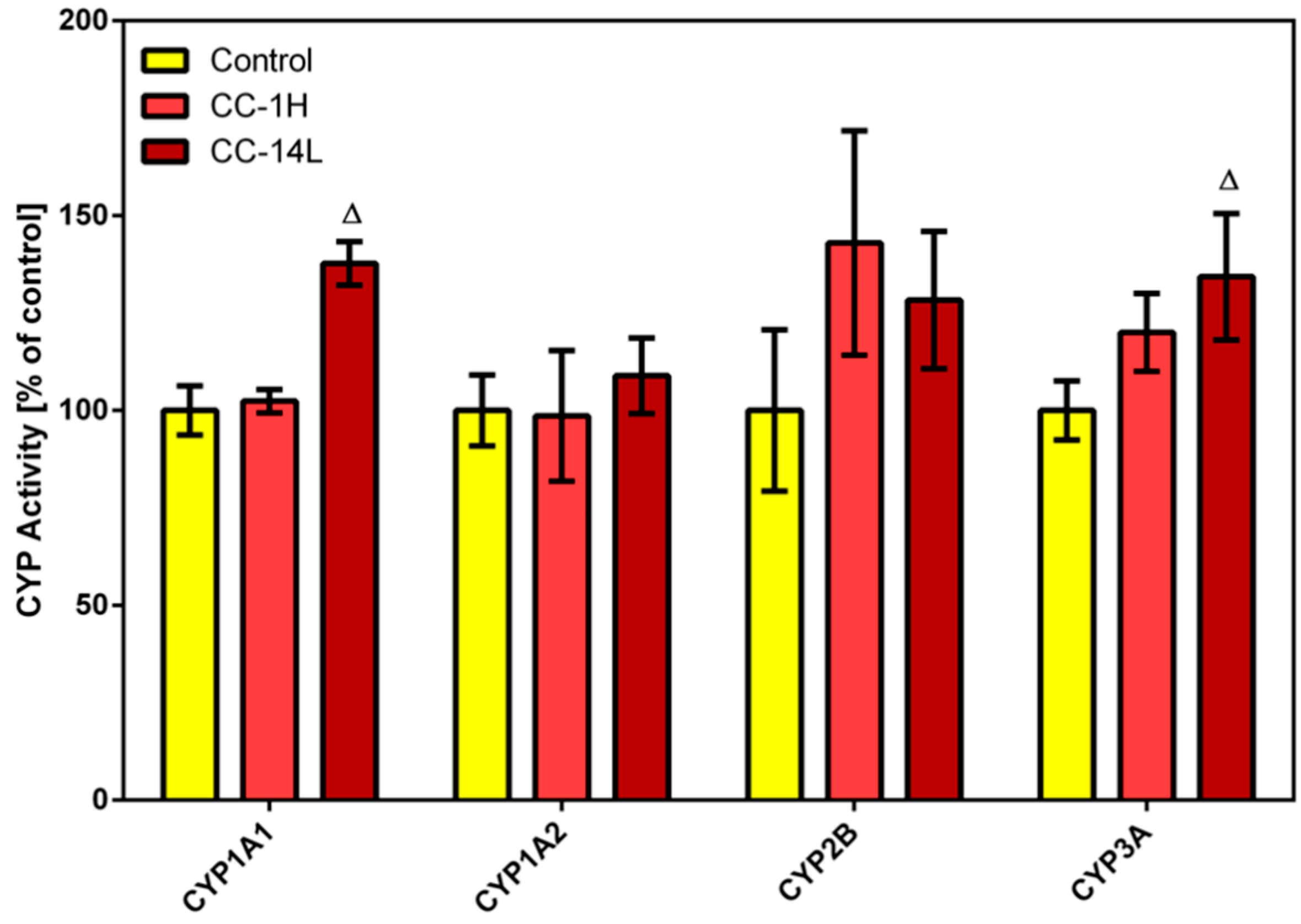
In this study, specific activities of four CYP isoforms (
i.e., CYP1A1, 1A2, CYP2B, and CYP3A) were assessed in microsomal fractions of rat liver (
Figure 1) and small intestine using alkoxyresorufins as substrates. Long-term treatment with low dose of cranberry extract (CC-14L) caused increase in the catalytic activity of hepatic CYP1A1 and CYP3A by 38% and 34%, respectively. Non-significant elevation in hepatic CYP2B activity was observed in CC-14L as well as in CC-1H groups. In rat small intestine, no specific activities of selected CYP isoforms were detected either in control or in CC-treated rats.
Figure 1.
Effect of cranberry extract administered in two dosage schemes on the specific activities of cytochrome P450 isoforms 1A1, 1A2, 2B and 3A in rat liver microsomes. Specific activities were determined fluorimetrically using alkoxyresorufines as substrates. Data represent the mean ± SD of three independent experiments. The triangle indicates a significant difference from the control (p < 0.05).
Figure 1.
Effect of cranberry extract administered in two dosage schemes on the specific activities of cytochrome P450 isoforms 1A1, 1A2, 2B and 3A in rat liver microsomes. Specific activities were determined fluorimetrically using alkoxyresorufines as substrates. Data represent the mean ± SD of three independent experiments. The triangle indicates a significant difference from the control (p < 0.05).
While in a study using healthy volunteers cranberry juice/extract caused inhibition of CYP3A [
11], our results showed mild increases in CYP1A1 and CYP3A activities. This discrepancy may be caused by the different experimental models. Moreover, cranberry extract also contains other constituents such as the flavonoid quercetin and the natural stilbene resveratrol, whose
in vivo inductive effects on CYP3A activity were described [
22,
23]. As the CYP3A subfamily is involved in the oxidative biotransformation of numerous drugs, modulation of CYP3A activity can have profound clinical consequences, but only with drugs that have narrow therapeutic windows [
24]. Induction of CYP1A1/2 is also considered to be undesirable as these isoforms carry out bioactivation of polycyclic aromatic hydrocarbons (e.g., benzo[a]pyrene), recognized carcinogens in humans and rodents. Fortunately, induction of CYP1A1/2 and CYP3A by cranberry extract was only mild and thus serious consequences could be excluded.
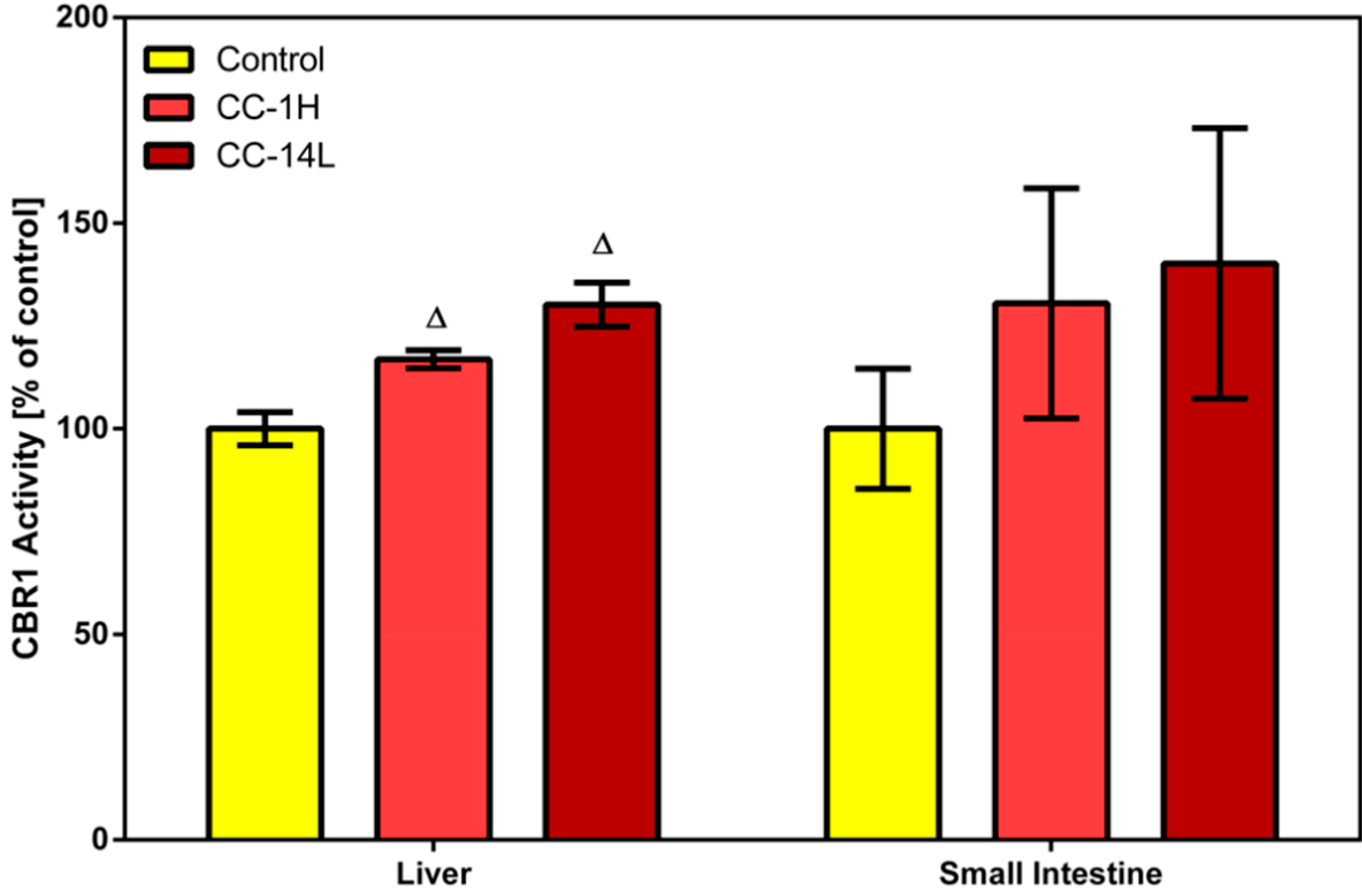
Phase I reducing reactions of xenobiotics are often catalyzed by carbonyl reductase 1, a member of the short-chain dehydrogenases/reductases family, that reduces a wide variety of carbonyl compounds including quinones, prostaglandins, menadione, and various xenobiotics [
24]. In our study, specific activity of CBR1 was measured in cytosolic fractions of liver and small intestinal mucosa homogenates using menadione as a specific substrate. Treatment of rats with CystiCran
® with both dosage schemes caused an elevation in CBR1 activities in the liver and small intestine, although only the changes found in the liver were statistically significant. Activity of hepatic CBR1 was increased by 17% and 30% in the CC-1H and CC-14L groups compared to untreated control, respectively (
Figure 2).
Figure 2.
Effect of cranberry extract administered in two dosage schemes on the specific activity of carbonyl reductase 1 in the cytosolic fractions of rat liver and small intestine. Specific activities were determined fluorimetrically using menadione as a substrate. Data represent the mean ± SD of three independent experiments. Triangles indicate a significant difference from the control (p < 0.05).
Figure 2.
Effect of cranberry extract administered in two dosage schemes on the specific activity of carbonyl reductase 1 in the cytosolic fractions of rat liver and small intestine. Specific activities were determined fluorimetrically using menadione as a substrate. Data represent the mean ± SD of three independent experiments. Triangles indicate a significant difference from the control (p < 0.05).
CBRs, particularly isoform CBR1, are leading biotransformation enzymes catalyzing deactivation of carbonyl-bearing drugs, including cytostatics. Therefore, regulation of their activity has been intensively studied and by this way, several flavonoids (e.g., quercetin, quercitrin and rutin) and anthocyanidins (e.g., delphinidin, cyanidin and malvidin) have been recognized as
in vitro inhibitors of CBR1 [
15,
25]. To our knowledge, no
in vivo studies covering this topic have been performed yet. However, these compounds may act as inducers of CBR1 or may not to influence its activity
in vivo. Their inductive/inhibitory effect is dependent also on the concentration used [
26,
27].
2.2. Effect of CystiCran® on the Activities of Phase II Conjugating Enzymes
In phase II, xenobiotics or their phase I metabolites undergo conjugation reactions with endogenous compounds such as glutathione or UDP-glucuronic acid. Conjugation usually introduces hydrophilic ionizable functional groups onto the molecule of a xenobiotic, thus making it more polar and facilitating its renal excretion. Almost all phase II biotransformation reactions lead to detoxification/deactivation of the parent xenobiotic or metabolites formed in phase I. Glucuronidation, catalyzed by UGT, is the most common conjugation pathway in humans. Typical UGT substrates are xenobiotic or eobiotic alcohols, phenols or carboxylic acids. On the other hand, electrophilic compounds are mostly conjugated with glutathione through the action of GSTs [
28].
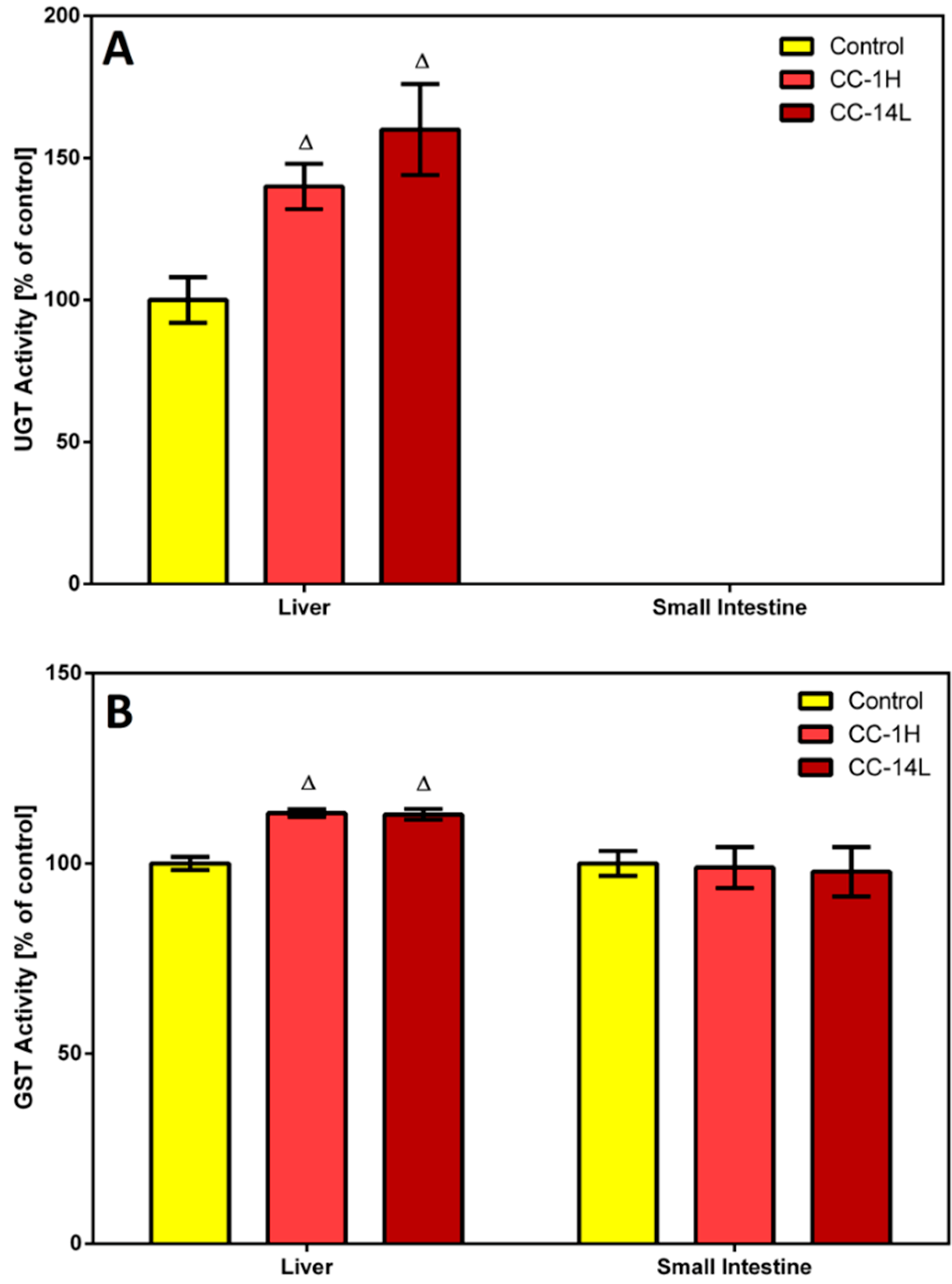
In this study, homogenates of rat liver and small intestine were analyzed for UGT and GST activities. Specific activity of UGT was tested in microsomal fractions using
p-nitrophenol as a substrate. In the liver, UGT activity was significantly increased in CC-treated rats compared to the control group (
Figure 3A).
Figure 3.
Effect of cranberry extract administered in two dosage schemes on the specific activities of UDP-glucuronosyl transferase in the microsomal fractions of rat liver and small intestine (A) and glutathione S-transferase in the cytosolic fractions of rat liver and small intestine (B). Specific activities were determined spectrophotometrically using p-nitrophenol and CDNB as UGT and GST substrates, respectively. Data represent the mean ± SD of three independent experiments. Triangles indicate a significant difference from the control (p < 0.05).
Figure 3.
Effect of cranberry extract administered in two dosage schemes on the specific activities of UDP-glucuronosyl transferase in the microsomal fractions of rat liver and small intestine (A) and glutathione S-transferase in the cytosolic fractions of rat liver and small intestine (B). Specific activities were determined spectrophotometrically using p-nitrophenol and CDNB as UGT and GST substrates, respectively. Data represent the mean ± SD of three independent experiments. Triangles indicate a significant difference from the control (p < 0.05).
On the other hand, specific UGT activity in the small intestine was at the detection limit, therefore no changes in this activity were detected. Catalytic activity of GST was assessed in cytosolic fractions by measuring its conjugation activity with the universal substrate 1-chloro-2,4-dinitrobenzene (CDNB). GST activity was elevated by 13% in the liver of both CC-treated groups compared to the control group, while an insignificant decrease in GST activity was observed in the small intestine of rats treated with CC (
Figure 3B).
Several constituents found in cranberry may be responsible for increased GST and UGT activities in rat liver. Consumption of various fruits and plants, which are rich sources of anthocyanidins as well as proanthocyanidins, was shown to elevate UGT and GST activities in rat liver [
29,
30]. Observed induction of UGT activity may be caused also by quercetin, whose content in cranberry is estimated to be in the range of 83–121 mg/kg (
i.e., about 50 µg per 0.5 mg cranberry supplement capsule) [
31]. This assumption was confirmed by the study of van der Logt
et al. [
32], in which significant induction of
p-nitrophenol glucuronidation was found in rat liver and small intestine after 14-day intake of quercetin. Another constituent capable of UGT and GST activities induction is the stilbene resveratrol. This compound caused 1.8-fold and 1.3-fold increases of UGT and GST activities in the liver of male rats upon 28-day intake of resveratrol, respectively [
33]. Moreover, 28-day administration of resveratrol to healthy volunteers led to induction of GSTP protein levels and UGT1A1 activity in individuals with low baseline enzyme level/activity, while overall GST and UGT1A1 activities were minimally affected by this intervention [
22]. Induction of GST activity by cranberry extract can be considered protective, because increased levels/activity of GST, especially in the gastrointestinal tract, can prevent organisms against harmful electrophiles [
26].
Certainly, it is necessary to take into consideration that the observed effects of cranberry extract may not be ascribed to only one chemical constituent as it is a complex mixture of various phytonutrients. Individual components of this mixture may interact with each other and the resulting biological activity of the cranberry extract is therefore dependent on their synergistic/antagonistic behavior. Moreover, biological effects of cranberry juice/extract are concentration-dependent. Thus, its inhibitory activity observed in vitro need not to be found also in vivo because the concentrations of active compounds in organism is influenced by their bioavailability. While high concentrations of bioactive compounds directly influence the activities of the studied enzymes in the in vitro experiments, the activity as well as expression of these enzymes are affected by low concentrations of these compounds in the in vivo study.
3. Experimental Section
3.1. Chemicals and Reagents
Benzyloxyresorufin, 7-ethoxyresorufin, 7-methoxyresorufin, 7-pentoxyresorufin, menadione, 1-chloro-2,4-dinitrobenzene (CDNB), 4-nitrophenol (NP), reduced glutathione (GSH), UDP-glucuronic acid, NADPH, and chemicals used for realization of electrophoresis were products of Sigma-Aldrich (Prague, Czech Republic). Precision Plus molecular weight standard and non-fat dry milk were purchased from Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA). For immunoblotting, rabbit polyclonal anti-UGT antibody (Cell Signaling, Leiden, The Netherlands), rabbit polyclonal anti-GST alpha antibody, rabbit monoclonal anti-CBR1 antibody, rabbit polyclonal anti-calnexin antibody, rabbit polyclonal anti-beta actin antibody (Abcam, Cambridge, UK), rabbit polyclonal anti-CYP1A1 antibody (Novus Biologicals, Cambridge, UK), rabbit polyclonal anti-CYP 3A4 antibody (Sigma-Aldrich), bovine anti-rabbit IgG antibody conjugated with horseradish peroxidase and chemiluminescence kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. All other chemicals used were of HPLC or analytical grade.
3.2. Laboratory Animals
Male Wistar rats were obtained from Meditox (Konarovice, Czech Republic). They were housed in air-conditioned animal quarters with a 12 h light/dark cycle. Food (a standard rat chow diet) and water were provided ad libitum. The animal protocols used in this work were evaluated and approved by the Ethic Committee of the Ministry of Education, Youth and Sports (Protocol 20363/2011-30). They are in accordance with the Guide for the Care and Use of Laboratory Animals (Protection of Animals from Cruelty Act No. 246/92, Czech Republic). At 12 weeks of age, rats were randomly divided into three groups of four. Rats of the first group (CC-14L) were orally administered with therapeutic dose of CystiCran® once daily for a period of 2 weeks; three times higher dose of CystiCran® was administered at once by gastric gavage to the second group of rats (CC-1H; 24 h before the end of experiment), and the third group represents untreated controls. At the end of experiment, animals were sacrificed by decapitation. Livers and small intestines were removed immediately, the intestinal contents were washed out with cold 0.9% saline solution and mucosa was scraped. Both tissues were stored at −80 °C until preparation of subcellular fractions.
3.3. Preparation of Microsomal and Cytosolic Fractions
Frozen liver or mucosa from small intestine were thawed at room temperature up to 15 min and processed to microsomal and cytosolic fractions. The subcellular fractions were isolated by differential centrifugation of the tissue homogenate [
35] and stored at −80 °C. Protein concentrations were assayed using the bicinchoninic acid (BCA) assay according to manufacturer's instructions (Sigma-Aldrich).
3.4. Enzyme Assays
Enzyme activities were assayed in the cytosolic and microsomal fractions from homogenates of liver and small intestinal mucosa of control and treated rats. The enzyme assays (each performed in 4–8 replicates) were repeated three times. The amount of organic solvents in the final reaction mixtures did not exceed 1% (v/v).
The activities of several CYP isoforms were assessed. The activities of 7-ethoxyresorufin
O-dealkylase (EROD; specific for CYP1A1), 7-methoxyresorufin
O-dealkylase (MROD; CYP1A2), 7-pentoxyresorufin
O-dealkylase (PROD; CYP2B) and 7-benzyloxyresorufin
O-dearylase (BROD; CYP3A) were measured at 37 °C using fluorimetric determination of arising resorufin [
36]. Each substrate dissolved in dimethylsulphoxide (DMSO) was added at a final concentration of 5 µM. Assays were conducted at the excitation/emission wavelengths of 530/585 nm using luminescence spectrophotometer LS50B (Perkin-Elmer, Cambridge, UK).
Carbonyl reductase 1 activity was measured in the cytosolic fractions using menadione as a substrate [
37]. Consumption of NADPH was determined at excitation/emission wavelength of 380/460 nm using a Perkin Elmer LS50B luminescence spectrophotometer at 37 °C.
The cytosolic glutathione S-transferase activities were assessed by standard colorimetric assay using CDNB as an electrophilic substrate [
38], which was adapted for measurement in 96-well plates. The absorbance of rising product
S-(2,4-dinitrophenyl)glutathione was detected at 340 nm by Tecan Infinite M200 multimode microplate reader (Tecan Group, Männedorf, Switzerland).
The microsomal UDP-glucuronosyltransferase activities towards
p-nitrophenol were assayed as described by Mizuma
et al. [
39]. Absorbance of unconjugated
p-nitrophenol was measured at 405 nm by the Tecan Infinite M200.
3.5. Western Blotting
Microsomal proteins of rat liver were separated by SDS-PAGE (10% stacking gel) [
40] and subsequently transferred onto nitrocellulose membranes (0.45 μm) using Trans-Blot
® TurboTM Transfer System (Bio-Rad, Hercules, CA, USA). Protein concentrations were determined using the BCA protein assay (Sigma-Aldrich). The membranes were blocked in 5% non-fat dry milk/TBS-Tween-20 for 2 h. Immunodetection of biotransformation enzymes was performed using corresponding primary antibodies (described in the Chemicals and Reagents section). The bands were visualized with respective horseradish peroxidase-conjugated secondary antibodies using the chemiluminescence kit according to manufacturer's instructions. Calnexin and β-actin served as the loading controls for microsomal and cytosolic fraction, respectively. Intensity of bands was evaluated using a C-DiGit
TM Blot Scanner (Li-Cor, Lincoln, NE, USA).
3.6. Statistical Analysis
All calculations were done using Microsoft Excel and GraphPad Prism 6. All values were expressed as mean ± SD. One-way Anova was used for the statistical evaluation of differences between control and treated groups, and differences were considered as significant when p < 0.05.