Inhibitory Effects of Colocasia esculenta (L.) Schott Constituents on Aldose Reductase
Abstract
:1. Introduction
2. Results and Discussion
| Extract and Fraction | Concentration (µg/mL) | Aldose Reductase Inhibition (%) | IC50 (μg/mL) |
|---|---|---|---|
| 95% EtOH extract | 10 | 43.66 ± 0.41 | - |
| Hexane fr. | 10 | 29.11 ± 0.31 | - |
| CHCl3 fr. | 10 | 13.05 ± 0.12 | - |
| EtOAc fr. | 5 | 74.89 ± 1.17 | 2.07 ± 0.02 |
| 2.5 | 54.83 ± 1.27 | ||
| 1 | 30.78 ± 0.64 | ||
| n-BuOH fr. | 10 | 84.02 ± 0.97 | 5.05 ± 0.55 |
| 5 | 51.57 ± 0.53 | ||
| 1 | 20.33 ± 1.15 | ||
| Water fr. | 10 | 24.3 ± 0.64 | - |
| Quercetin a | 2.5 | 69.64 ± 0.72 | 1.09 ± 0.01 |
| 1 | 48.9 ± 0.51 | ||
| 0.5 | 31.62 ± 0.09 |
| Compounds | Concentration (µg/mL) | Inhibition (%) | IC50 (µg/mL) | IC50 (µM) | |
|---|---|---|---|---|---|
| Quercetin a | 5 | 81.14 ± 0.81 | 1.25 ± 0.05 | 4.12 ± 0.16 | |
| 1 | 48.60 ± 1.44 | ||||
| 0.5 | 26.32 ± 0.59 | ||||
| 1 | Tryptophan | 10 | 19.99 ± 2.01 | - | - |
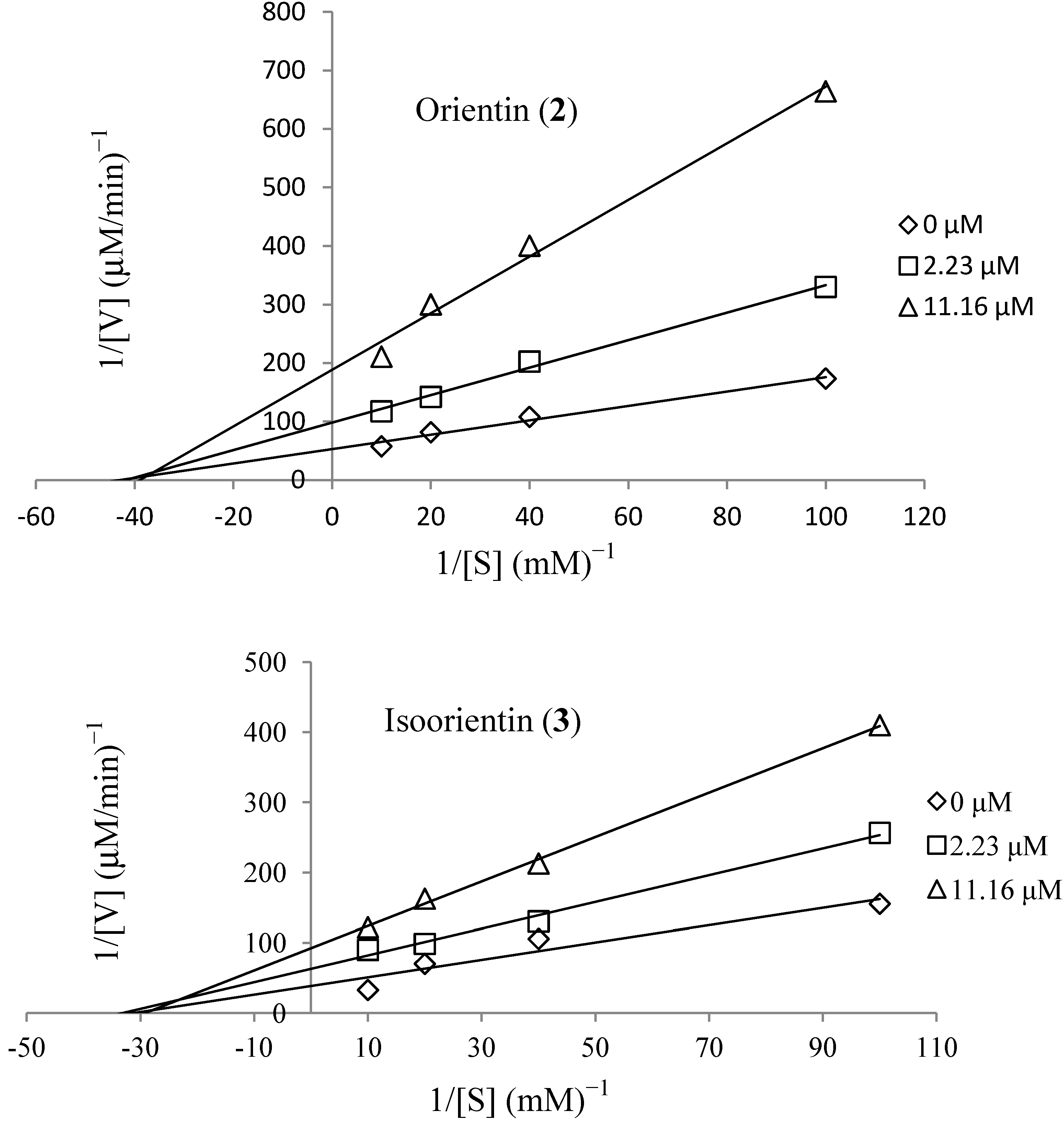
| 2 | Orientin | 5 | 93.97 ± 0.98 | 0.74 ± 0.03 | 1.65 ± 0.07 |
| 1 | 64.57 ± 0.52 | ||||
| 0.5 | 35.29 ± 0.36 | ||||
| 3 | Isoorientin | 5 | 77.73 ± 0.77 | 0.86 ± 0.05 | 1.92 ± 0.11 |
| 1 | 57.64 ± 0.58 | ||||
| 0.5 | 37.23 ± 0.37 | ||||
| 4 | Vitexin | 10 | 48.53 ± 0.87 | - | - |
| 5 | Isovitexin | 10 | 76.59 ± 0.74 | 3.18 ± 0.31 | 7.36 ± 0.72 |
| 5 | 60.29 ± 0.61 | ||||
| 1 | 23.34 ± 0.25 | ||||
| 6 | Luteolin-7-O-glucoside | 10 | 76.24 ±0.77 | 3.35 ± 0.32 | 7.47 ± 0.71 |
| 5 | 61.88 ± 0.60 | ||||
| 1 | 19.06 ± 0.21 | ||||
| 7 | Luteolin-7-O-rutinoside | 5 | 72.69 ± 0.73 | 1.54 ± 0.14 | 2.59 ± 0.26 |
| 1 | 40.31 ± 0.40 | ||||
| 0.5 | 29.91 ± 0.30 | ||||
| 8 | Rosmarinic acid | 5 | 87.33 ± 0.87 | 2.99 ± 0.27 | 5.38 ± 0.75 |
| 1 | 62.79 ± 0.60 | ||||
| 0.5 | 18.34 ± 0.19 | ||||
| 9 | 1-O-Feruloyl-d-glucoside | 10 | 4.06 ± 0.40 | - | - |
| 10 | 1-O-Caffeoyl-d-glucoside | 10 | 73.19 ± 0.99 | 4.84 ± 0.47 | 14.14 ± 0.14 |
| 5 | 55.57 ± 0.78 | ||||
| 2.5 | 24.72 ± 0.36 | ||||
| Compounds | Rat Erythrocyte Galactitol Content (µM) a | Galactitol Content µg/lens Wet Weight (g) b |
|---|---|---|
| Galactitol free | - | 3.13 ± 0.06 |
| Control | 86.70 ± 1.61 | 250.47 ± 5.01 |
| Quercetin c | 45.17 ± 0.81 | 241.07 ± 4.57 |
| Isoorientin | 56.18 ± 1.00 | 243.14 ± 4.71 |
| Orientin | 72.72 ± 0.98 | 227.89 ± 4.52 |
| Extract and Fraction | Concentration (µg/mL) | Inhibition (%) | IC50 (µg/mL) | IC50 (µM) | |
|---|---|---|---|---|---|
| Trolox a | 8.33 | 92.92 ± 0.06 | 2.87 ± 0.01 | 11.49 ± 0.04 | |
| 3.33 | 57.40 ± 2.03 | ||||
| 1.67 | 27.07 ± 1.15 | ||||
| Quercetin b | 1.67 | 81.36 ± 1.75 | 0.97 ± 0.04 | 3.20 ± 0.12 | |
| 0.83 | 42.49 ± 3.39 | ||||
| 0.33 | 22.98 ± 3.55 | ||||
| 95% EtOH Extract | 10 | 26.07 ± 0.61 | - | - | |
| Hexane fr. | 33.33 | 14.77 ± 0.85 | - | - | |
| CHCl3 fr. | 33.33 | 42.4 ± 1.13 | - | - | |
| EtOAc fr. | 16.67 | 71.10 ± 3.62 | 10.86 ± 0.84 | - | |
| 8.33 | 41.64 ± 3.10 | ||||
| 3.33 | 21.78 ± 2.11 | ||||
| n-BuOH fr. | 33.33 | 48.35 ± 1.07 | - | - | |
| Water fr. | 33.33 | 9.90 ± 0.60 | - | - | |
| Compounds | Concentration (µg/mL) | Inhibition (%) | IC50 (µg/mL) | IC50 (µM) |
|---|---|---|---|---|
| Trolox a | 8.33 | 92.92 ± 0.06 | 2.87 ± 0.01 | 11.49 ± 0.04 |
| 3.33 | 57.40 ± 2.03 | |||
| 1.67 | 27.07 ± 1.15 | |||
| Quercetin b | 1.67 | 81.36 ± 1.75 | 0.97 ± 0.04 | 3.20 ± 0.12 |
| 0.83 | 42.49 ± 3.39 | |||
| 0.33 | 22.98 ± 3.55 | |||
| Tryptophan | 16.67 | 37.51 ± 2.93 | - | - |
| Orientin | 8.33 | 72.55 ± 4.10 | 5.59 ± 0.42 | 12.47 ± 0.95 |
| 3.33 | 31.76 ± 3.19 | |||
| 1.67 | 17.56 ± 2.21 | |||
| Isoorientin | 8.33 | 60.92 ± 2.81 | 6.53 ± 0.53 | 14.55 ± 1.18 |
| 3.33 | 31.69 ± 4.94 | |||
| 1.67 | 17.87 ± 3.08 | |||
| Vitexin | 16.67 | 46.17 ± 2.65 | - | - |
| Isovitexin | 16.67 | 44.40 ± 4.91 | - | - |
| Luteolin-7-O-glucoside | 8.33 | 52.11 ± 3.98 | 8.00 ± 0.68 | 17.84 ± 1.51 |
| 3.33 | 22.66 ± 2.47 | |||
| 1.67 | 13.49 ± 1.09 | |||
| Luteolin-7-O–rutinoside | 16.67 | 79.82 ± 0.67 | 9.38 ± 0.47 | 15.77 ± 0.80 |
| 8.33 | 49.55 ± 3.91 | |||
| 3.33 | 21.44 ± 1.46 | |||
| Rosmarinic acid | 8.33 | 60.25 ± 4.66 | 6.91 ± 0.61 | 19.18 ± 1.69 |
| 3.33 | 23.61 ± 2.27 | |||
| 1.67 | 16.20 ± 3.85 | |||
| 1-O-Feruloyl-d-glucoside | 16.67 | 59.10 ± 2.55 | 13.31 ± 0.23 | 37.35 ± 0.64 |
| 8.33 | 35.36 ± 1.54 | |||
| 3.33 | 13.51 ± 1.62 | |||
| 1-O-Caffeoyl-d-glucoside | 8.33 | 50.87 ± 1.30 | 8.04 ± 0.49 | 23.47 ± 1.43 |
| 3.33 | 29.84 ± 1.19 | |||
| 1.67 | 14.07 ± 0.39 |
3. Experimental
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Preparation of Aldose Reductase
3.5. Determination of Aldose Reductase Inhibition in Vitro
3.6. Kinetics of Recombinant Human Aldose Reductase
3.7. Lens Culture and Intracellular Galactitol Measurement
3.8. Blood Culture and Intracellular Galactitol Measurement
3.9. ABTS+ Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar]
- Moon, H.I.; Jung, J.C.; Lee, J. Aldose reductase inhibitory effect by tectorigenin derivatives from viola hondoensis. Bioorg. Med. Chem. 2006, 14, 7592–7594. [Google Scholar] [CrossRef]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin. Phytother. Res. 2013, 28, 317–333. [Google Scholar] [CrossRef]
- TomLinson, D.R.; Stevens, E.J.; Diemel, L.T. Aldose reductase inhibitors and their potential for the treatment of diabetic complications. Trends Pharmacol. Sci. 1994, 15, 293–297. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Matsuda, H.; Murakami, T.; Yashiro, K.; Yamahara, J.; Yoshikawa, M. Antidiabetic principles of natural medicines. IV. Aldose reductase and qlpha-glucosidase inhibitors from the roots of Salacia oblonga Wall. (Celastraceae): Structure of a new friedelane-type triterpene, kotalagenin 16-acetate. Chem. Pharm. Bull. 1999, 47, 1725–1729. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, H.; Nishida, N.; Li, Y.; Toguchida, I.; Yamahara, J.; Matsuda, H. Antidiabetic principles of natural medicines. II. Aldose reductase and alpha-glucosidase inhibitors from Brazilian natural medicine, the leaves of Myrcia multiflora DC. (Myrtaceae): Structures of myrciacitrins I and II and myrciaphenones A and B. Chem. Pharm. Bull. 1998, 46, 113–119. [Google Scholar] [CrossRef]
- Patel, M.B.; Mishra, S. Isoquinoline alkaloids from tinospora cordifolia inhibit rat lens aldose reductase. Phytother. Res. 2012, 26, 1342–1347. [Google Scholar] [CrossRef]
- Jung, H.A.; Yoon, N.Y.; Kang, S.S.; Kim, Y.S.; Choi, J.S. Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J. Pharm. Pharmacol. 2008, 60, 1227–1236. [Google Scholar] [CrossRef]
- Kawanishi, K.; Ueda, H.; Moriyasu, M. Aldose reductase inhibitors from the nature. Curr. Med. Chem. 2003, 10, 1353–1374. [Google Scholar] [CrossRef]
- Manzanaro, S.; Salva, J.; de la Fuente, J.A. Phenolic marine natural products as aldose reductase inhibitors. J. Nat. Prod. 2006, 69, 1485–1487. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Kaensombath, L.; Lindberg, J.E. Effect of replacing soybean protein by taro leaf (Colocasia esculenta (L.) Schott) protein on growth performance of exotic (Landrace x Yorkshire) and native (Moo Lath) Lao pigs. Trop. Anim. Health Proc. 2012, 45, 45–51. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohyd. Polym. 2012, 90, 1204–1209. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Iroaganachi, M.; Eleazu, K.C. Ameliorative potentials of cocoyam (Colocasia esculenta L.) and unripe plantain (Musa paradisiaca L.) on the relative tissue weights of streptozotocin-induced diabetic rats. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef]
- Cambie, R.C.; Ferguson, L.R. Potential functional foods in the traditional Maori diet. Mutat. Res. 2003, 523–524, 109–117. [Google Scholar] [CrossRef]
- Rao, A.R.; Veeresham, C.; Asres, K. In vitro and in vivo inhibitory activities of four Indian medicinal plant extracts and their major components on rat aldose reductase and generation of advanced glycation endproducts. Phytother. Res. 2013, 27, 753–760. [Google Scholar] [CrossRef]
- Jain, V.; Viswanatha, G.L.; Manohar, D.; Shivaprasad, H.N. Isolation of antidiabetic principle from fruit rinds of Punica granatum. Evid.-Based Complement. Altern. 2012, 2012. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z. A new coumarin glycoside from Glehnia littoralis. Yao Xue Xue Bao 2007, 42, 1070–1073. [Google Scholar]
- Yan, C.; Lin, L.; Liu, H.; Lin, Z.; Chen, P.; Cai, C.; Zheng, L. Study of flavonoids from leaves of Santalum album. Zhongguo Zhong Yao Za Zhi 2011, 36, 3130–3133. [Google Scholar]
- Chen, H.Y.; Zhou, C.X.; Lou, Y.J.; Duan, Z.H.; Zhao, Y. Chemical constituents from Elsholtzia blanda. Zhongguo Zhong Yao Za Zhi 2005, 30, 1589–1591. [Google Scholar]
- Zhang, C.; Yin, Z.; Ye, W.; Guan, Y.; Guo, L.; Zhang, J.; Shen, W. Chemical constituents from stems of Lonicera japonica. Zhongguo Zhong Yao Za Zhi 2009, 34, 3051–3053. [Google Scholar]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against alpha-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef]
- Du, Q.; Xu, Y.; Li, L.; Zhao, Y.; Jerz, G.; Winterhalter, P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. J. Agric. Food Chem. 2006, 54, 4186–4190. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, J.M.; Lee, H.J.; Kim, C.Y.; Lee, E.H.; Um, B.H. Aldose reductase and advanced glycation endproducts inhibitory effect of Phyllostachys nigra. Biol. Pharm. Bull. 2007, 30, 1569–1572. [Google Scholar] [CrossRef]
- Varma, S.D.; Kinoshita, J.H. Inhibition of lens aldose reductase by flavonoids—Their possible role in the prevention of diabetic cataracts. Biochem. Pharm. 1976, 25, 2505–2513. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 788–795. [Google Scholar] [CrossRef]
- Islam, M.N.; Choi, S.H.; Moon, H.E.; Park, J.J.; Jung, H.A.; Woo, M.H.; Woo, H.C.; Choi, J.S. The inhibitory activities of the edible green alga Capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur. J. Nutr. 2013, 53, 233–242. [Google Scholar]
- Kim, T.H.; Kim, J.K.; Kang, Y.H.; Lee, J.Y.; Kang, I.J.; Lim, S.S. Aldose reductase inhibitory activity of compounds from Zea mays L. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.K.; Bae, Y.S.; Won, M.H.; Kang, I.J.; Lim, S.S. Inhibitory effect of glucodistylin from the bark of Quercus acutissima on human recombinant aldose reductase and sorbitol accumulation. Arch. Pharm. Res. 2011, 34, 211–215. [Google Scholar]
- Yoon, H.N.; Lee, M.Y.; Kim, J.K.; Suh, H.W.; Lim, S.S. Aldose reductase inhibitory compounds from Xanthium strumarium. Arch. Pharm. Res. 2013, 36, 1090–1095. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circulation Res. 2000, 87, 840–844. [Google Scholar]
- Hayman, S.; Kinoshita, J.H. Isolation and properties of lens aldose reductase. J. Biol. Chem. 1965, 240, 877–882. [Google Scholar]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef]
- Lim, S.S.; Jung, Y.J.; Hyun, S.K.; Lee, Y.S.; Choi, J.S. Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens. Phytother. Res. 2006, 20, 825–830. [Google Scholar] [CrossRef]
- De la Fuente, J.A.; Manzanaro, S. Aldose reductase inhibitors from natural sources. Nat. Prod. Rep. 2003, 20, 243–251. [Google Scholar] [CrossRef]
- Kwang-Hyok, S.; Ui-Nam, P.; Sarkar, C.; Bhadra, R. A sensitive assay of red blood cell sorbitol level by high performance liquid chromatography: Potential for diagnostic evaluation of diabetes. Clin. Chim. Acta 2005, 354, 41–47. [Google Scholar] [CrossRef]
- Shinohara, R.; Ohta, Y.; Yamauchi, M.; Ishiguro, I. Improved fluorometric enzymatic sorbitol assay in human blood. Clin. Chim. Acta 1998, 273, 171–184. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Sample Availability: Samples of the compounds 2 and 3 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.M.; Hwang, S.H.; Kang, B.G.; Hong, J.S.; Lim, S.S. Inhibitory Effects of Colocasia esculenta (L.) Schott Constituents on Aldose Reductase. Molecules 2014, 19, 13212-13224. https://doi.org/10.3390/molecules190913212
Li HM, Hwang SH, Kang BG, Hong JS, Lim SS. Inhibitory Effects of Colocasia esculenta (L.) Schott Constituents on Aldose Reductase. Molecules. 2014; 19(9):13212-13224. https://doi.org/10.3390/molecules190913212
Chicago/Turabian StyleLi, Hong Mei, Seung Hwan Hwang, Beom Goo Kang, Jae Seung Hong, and Soon Sung Lim. 2014. "Inhibitory Effects of Colocasia esculenta (L.) Schott Constituents on Aldose Reductase" Molecules 19, no. 9: 13212-13224. https://doi.org/10.3390/molecules190913212





