Synthesis and Cytotoxic Evaluation of a Series of 2-Amino-Naphthoquinones against Human Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
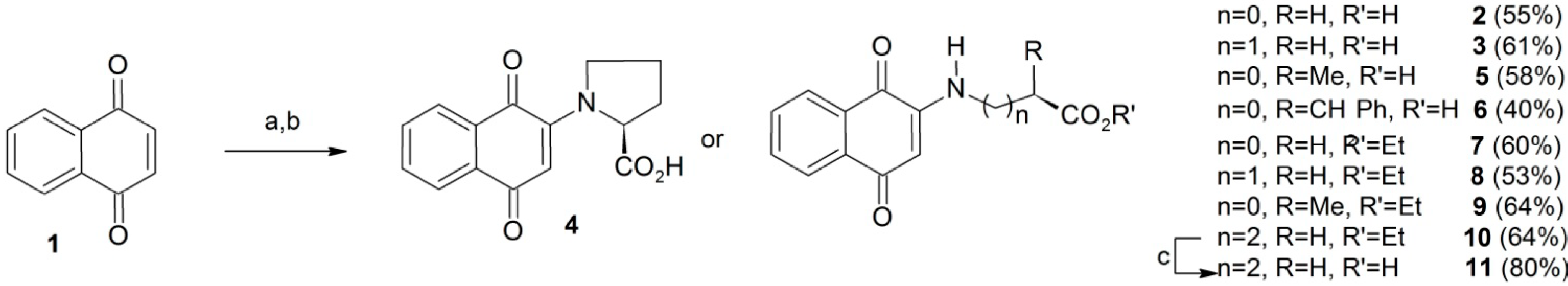
| HCT-8 | GI% | MDAMB-435 | GI% | SF-295 | GI% | |
|---|---|---|---|---|---|---|
| ANPQ | average | SD | Average | SD | average | SD |
| 2 | 81.65 | 3.15 | 100.00 | 0.06 | 81.13 | 3.26 |
| 3 | 56.04 | 0.54 | 26.36 | 33.11 | 55.95 | 0.15 |
| 4 | 59.06 | 57.01 | 36.74 | 70.56 | 59.33 | 56.49 |
| 5 | 84.08 | 1.18 | 100.00 | 0.12 | 83.79 | 1.12 |
| 6 | 86.13 | 2.41 | 100.00 | 0.09 | 86.06 | 2.60 |
| 7 | 99.51 | 1.28 | 100.00 | 0.15 | 99.50 | 1.22 |
| 8 | 28.17 | 17.64 | 11.67 | 53.27 | 32.71 | 17.12 |
| 9 | 75.36 | 0.15 | 100.05 | 0.22 | 75.07 | 0.20 |
| 10 | 49.57 | 7.03 | 59.11 | 12.59 | 49.21 | 6.52 |
| 11 | 61.36 | 8.94 | 11.13 | 36.74 | 61.21 | 8.61 |
| HCT-8 | MDAMB-435 | SF-295 | HL-60 | HCT-116 | OVCAR-8 | NCI-H358M | PC3-M | PBMC | |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.89/3.84 | 1.21/5.23 | 0.57/2.46 | 0.7/3.02 | 1.31/5.66 | 3.89/16.78 | >5/21.57 | >5/21.57 | 5.51/23.77 |
| (0.81–0.99) | (0.87–1.69) | (0.41–0.77) | (0.49–1.02) | (1.12–1.54) | (3.52–4.30) | (4.27–7.1) | |||
| 5 | 0.83/3.38 | 0.49/1.99 | 0.63/2.56 | 1.05/4.27 | 1.11/4.52 | 2.36/9.61 | 0.90/3.36 | 1.61/6.55 | 7.34/29.89 |
| (0.71–0.96) | (0.42–0.58) | (0.52–0.76) | (0.87–1.26) | (0.94–1.29) | (1.92–2.91) | (0.64–1.29) | (1.46–1.78) | (6.15–8.7) | |
| 6 | 1.59/4.94 | 1.46/4.53 | 0.65/2.01 | 1.31/4.07 | 2.15/6.67 | 1.87/5.81 | 0.82/2.54 | 1.70/5.28 ) | 12.18/37.84 |
| (1.19–2.14) | (1.14–1.88) | (0.52–0.81) | (0.98–1.75) | (1.91–2.42) | (1.62–2.16 | (0.62–1.10) | (1.30–2.21 | (10.16–14.6) | |
| 7 | 1.84/7.09 | 1.33/5.12 | 0.83/3.19 | 1.24/4.77 | 2.13/8.20 | 2.00/7.70 | 0.64/2.46 | 1.63/6.28 | 9.00/34.67 |
| (1.64–2.06) | (1.05–1.17) | (0.72–0.95) | (1.12–1.37) | (1.76–2.57) | (1.70–2.35) | (0.48–0.85) | (1.37–1.94) | (7.73–10.51) | |
| 9 | 1.33/4.86 | 1.18/4.31 | 1.68/6.13 | 1.74/6.35 | 3.11/11.36 | 1.88/6.87 | 0.98/3.58 | 2.62/9.57 | 17.61/64.34 |
| (0.86–2.07) | (0.88–1.60) | (1.25–2.27) | (1.39–2.17) | (2.61–3.72) | (0.90–3.93) | (0.72–1.33) | (2.22–3.10) | (15.0–20.68) | |
| Dox | 0.07/0.13 | 0.48/0.83 | 0.23/0.83 | 0.02/0.03 | 0.01/0.02 | 0.26/0.45 | 0.9/1.65 | 0.20/0.36 | 0.23/0.42 |
| (0.03–0.12) | (0.34–0.66) | (0.19–0.25) | (0.01–0.02) | (0.01–0.02) | (0.17–0.31) | (0.6–1.6) | (0.17–0.23) | (0.10–0.38) |
3. Experimental Section
3.1. General Information
3.2. Synthesis
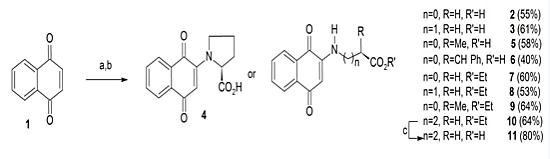
3.2.1. General Procedure for the Preparation of Compounds 2–6
 = +84.7 (C = 0.01, DMSO). Anal. Calcd. for C15H13NO4: C, 66.41; H, 4.83; N, 5.16. Found: C, 66.14; H, 4.85; N, 5.35.
= +84.7 (C = 0.01, DMSO). Anal. Calcd. for C15H13NO4: C, 66.41; H, 4.83; N, 5.16. Found: C, 66.14; H, 4.85; N, 5.35. = +14.4 (C = 0.01, DMSO). Anal. Calcd. for C13H11NO4: C, 63.67; H, 4.52; N, 5.71. Found: C, 61.75; H, 4.70; N, 5.70.
= +14.4 (C = 0.01, DMSO). Anal. Calcd. for C13H11NO4: C, 63.67; H, 4.52; N, 5.71. Found: C, 61.75; H, 4.70; N, 5.70. = +85.86 (C = 0.01, DMSO).
= +85.86 (C = 0.01, DMSO).3.2.2. General Procedure for the Preparation of Compounds 7–10
3.3. Cytotoxicity against Cancer Cell Lines
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paula, C.C.; de Mutti, C.F.; Padoin, S.M.M.; Bubadué, R.M.; Santos, E.E.P.; Silva, C.B. Palliative care in cancer: Literature review article. J. Nurs. UFPE Line 2013, 7, 246–261. [Google Scholar]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 221–247. [Google Scholar] [CrossRef]
- Camara, C.A.; Silva, T.M.S.; Silva, T.G.; Martins, R.M.; Barbosa, T.P.; Pinto, A.C.; Vargas, M.D. Molluscicidal activity of 2-hydroxy-[1,4]naphthoquinone and derivatives. An. Acad. Bras. Cienc. 2008, 80, 329–334. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Lemos, T.L.G.; de Mattos, M.C.; Segundo, T.A.; Santiago, G.M.P.; Braz-Filho, R. New enamine derivatives of lapachol and biological activity. An. Acad. Bras. Cienc. 2002, 74, 211–221. [Google Scholar] [CrossRef]
- Li, C.J.; Li, Y.Z.; Pinto, A.V.; Pardee, A.B. Potent inhibition of tumor survival in vivo by β-lapachone plus taxol: Combining drugs imposes different artificial checkpoints. Proc. Natl. Acad. Sci. USA 1999, 96, 13369–13374. [Google Scholar] [CrossRef]
- Silva, M.N.; Ferreira, V.F.; Souza, M. An overview of the chemistry and pharmacology of naphthoquinones with emphasis on β-lapachone and derivatives. Quim. Nova 2003, 29, 407–416. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; La Mont, J.T.; Pardee, A.B.; Chiang, J.L. Selective killing of cancer cells by beta-lapachone: Direct checkpoint activation as a strategy against cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 2674–2678. [Google Scholar] [CrossRef]
- Silva, T.M.; Camara, C.A.; Barbosa, T.P.; Soares, A.Z.; da Cunha, L.C.; Pinto, A.C.; Vargas, M.D. Molluscicidal activity of synthetic lapachol amino and hydrogenated derivatives. Bioorg. Med. Chem. 2005, 13, 193–196. [Google Scholar] [CrossRef]
- Lee, Y.R.; Choi, J.H.; Trinh, D.T.L.; Kim, N.W. A Concise Route for the Synthesis of Pyranonaphthoquinone Derivatives. Synthesis 2005, 18, 3026–3034. [Google Scholar]
- Chen, J.; Huang, Y.; Liu, G.; Afraziab, Z.; Sinn, E.; Padhye, S.; Ma, Y. The cytotoxicity and mechanisms of 1,2-naphthoquinone thiosemicarbazone and its metal derivatives against MCF-7 human breast cancer cells. Toxicol. Appl. Pharmacol. 2004, 197, 40–48. [Google Scholar] [CrossRef]
- Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Potent antitumor activity of synthetic 1,2-Naphthoquinones and 1,4-Naphthoquinones. Bioorg. Med. Chem. 2003, 11, 3179–3191. [Google Scholar] [CrossRef]
- Kongkathip, N.; Luangkamin, S.; Kongkathip, B.; Sangma, C.; Grigg, R.; Kongsaeree, P.; Prabpai, S.; Pradidphol, N.; Piyaviriyagul, S.; Siripong, P. Synthesis of Novel Rhinacanthins and Related Anticancer Naphthoquinone Esters. J. Med. Chem. 2004, 47, 4427–4438. [Google Scholar] [CrossRef]
- Bittner, S.; Gorohovsky, S.; Lozinsky, E.; Shames, A.I. EPR study of anion radicals of various N-quinonyl amino acids. Amino Acids 2000, 19, 439–449. [Google Scholar] [CrossRef]
- Shresta-Dawadi, P.B.; Bittner, S.; Fridkin, M.; Rahimipour, S. On the synthesis of naphthoquinonyl heterocyclic amino acids. Synthesis 1996, 16, 1468–1472. [Google Scholar]
- Mital, A.; Sonawane, M.; Bindal, S.; Mahlavat, S.; Negi, V. Substituted 1,4-naphthoquinones as a new class of antimycobacterial agents. Der Pharma Chemica 2010, 2, 63–73. [Google Scholar]
- Valente, C.; Moreira, R.; Guedes, R.C.; Iley, J.; Jaffar, M.; Douglas, K.T. The 1,4-naphthoquinone scaffold in the design of cysteine protease inhibitors. Bioorg. Med. Chem. 2007, 15, 5340–5350. [Google Scholar] [CrossRef]
- Gershon, H.; Shanks, L. Fungitoxicity of 1,4-naphthoquinones to Candida albicans and Trichophyton mentagrophytes. Can. J. Microbiol. 1976, 21, 1317–1321. [Google Scholar] [CrossRef]
- Pailard, F.; Finot, F.; Mouche, I.; Prenez, A.; Vericat, J.A. Use of primary cultures of rat hepatocytes to predict toxicity in the early development of new chemical entities. Toxicol. In Vitro 1999, 13, 693–700. [Google Scholar] [CrossRef]
- Freitas, H.P.S.; Maia, A.I.V.; Silveira, E.R.; Marinho-Filho, J.D.B.; Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V.; Pessoa, O.D.L. Cytotoxic cordiaquinones from the roots of Cordia polycephala. J. Braz. Chem. Soc. 2012, 23, 1558–1562. [Google Scholar] [CrossRef]
- Silva-Jr, E.N.; Cavalcanti, B.C.; Guimarães, T.T.; Pinto, M.C.; Cabral, I.O.; Pessoa, C.; Costa-Lotufo, L.V.; Moraes, M.O.; Andrade, C.K.; Santos, M.R.; et al. Synthesis and evaluation of quinonoid compounds against tumor cell lines. Eur. J. Med. Chem. 2011, 46, 399–410. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Montenegro, R.C.; Militao, G.C.G.; Fonseca, A.M.; Pessoa, O.D.; Lemos, T.L.G.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V. Bioactivity of biflorin, a typical o-naphthoquinone isolated from Capraria biflora L. Z. Naturforsch. 2005, 60, 394–398. [Google Scholar]
- Montenegro, R.C.; Araújo, A.J.; Molina, M.T.; Marinho-Filho, J.D.; Rocha, D.D.; Lopéz-Montero, E.; Goulart, M.O.; Bento, E.S.; Alves, A.P.; Pessoa, C.; et al. Cytotoxic activity of naphthoquinones with special emphasis on juglone and its 5-O-methyl derivative. Chemico-Biol. Interact. 2010, 184, 439–448. [Google Scholar] [CrossRef]
- Tung, H.N.; Du, G.J.; Wang, C.Z.; Yuan, C.S.; Shoyama, Y. Naphthoquinone Components from Alkanna tinctoria (L.) Tausch Show Significant Antiproliferative Effects on Human Colorectal Cancer Cells. Phytother. Res. 2013, 27, 66–70. [Google Scholar] [CrossRef]
- Neves, A.P.; da Silva, G.B.; Vargas, M.D.; Pinheiro, C.B.; Visentin, L.C.; Filho, J.D.M.B.; Araújo, A.J.; Costa-Lotufo, L.V.; Pessoa, C.; Moraes, M.O. Novel platinum(II) complexes of 3-(aminomethyl)naphthoquinone Mannich bases: Synthesis, crystal structure and cytotoxic activities. Dalton Trans. 2010, 39, 10203–10216. [Google Scholar] [CrossRef]
- Silva, M.G.; Camara, C.A.; Silva, T.M.S.; Feitosa, A.C.S.; Meira, A.S.; Pessoa, C. Synthesis of 2,3-diyne-1,4-naphthoquinone derivatives and evaluation of cytotoxic activity against tumor cell lines. J. Braz. Chem. Soc. 2013, 24, 1420–1426. [Google Scholar]
- Bittner, S.; Gorohovsky, S.; Paz-Tal, O.; Becker, J.Y. Synthesis, electrochemical and spectral properties of some ω-N-quinonyl amino acids. Amino Acids 2002, 22, 71–93. [Google Scholar] [CrossRef]
- Osman, A.M.; El-Maghraby, M.A.; Khalil, Z.H.; Hassan, K.M. Reactions of p-quinones with α-amino acids and their esters. Egyptian J. Chem. 1975, 18, 993–999. [Google Scholar]
- Braun, L.L.; Sumerford, W.T. Some 2-(2-carboxyethyl)amino-1,4-naphthoquinone derivatives. J. Chem. Eng. Data 1966, 11, 264–264. [Google Scholar] [CrossRef]
- Akiba, M.; Okuyama, M.; Kosugi, Y.; Takada, T. Photolysis of amino-substituted 1,4-naphthoquinones. Heterocycles 1977, 6, 1773. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, P.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesh, H.; Kenney, S.; Boyd, M.R.J. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica 1996, 4, 15–19. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Moraes, T.A.P.; Filha, M.J.S.; Camara, C.A.; Silva, T.M.S.; Soares, B.M.; Bomfim, I.S.; Pessoa, C.; Ximenes, G.C.; Silva, V.A., Jr. Synthesis and Cytotoxic Evaluation of a Series of 2-Amino-Naphthoquinones against Human Cancer Cells. Molecules 2014, 19, 13188-13199. https://doi.org/10.3390/molecules190913188
De Moraes TAP, Filha MJS, Camara CA, Silva TMS, Soares BM, Bomfim IS, Pessoa C, Ximenes GC, Silva VA Jr. Synthesis and Cytotoxic Evaluation of a Series of 2-Amino-Naphthoquinones against Human Cancer Cells. Molecules. 2014; 19(9):13188-13199. https://doi.org/10.3390/molecules190913188
Chicago/Turabian StyleDe Moraes, Thiago A. P., Maria J. S. Filha, Celso A. Camara, Tania M. S. Silva, Bruno M. Soares, Igor S. Bomfim, Claudia Pessoa, George C. Ximenes, and Valdemiro A. Silva, Jr. 2014. "Synthesis and Cytotoxic Evaluation of a Series of 2-Amino-Naphthoquinones against Human Cancer Cells" Molecules 19, no. 9: 13188-13199. https://doi.org/10.3390/molecules190913188