Comparative Pharmacokinetics Study of Sinomenine in Rats after Oral Administration of Sinomenine Monomer and Sinomenium Acutum Extract
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chromatographic Separations
2.2. Linearity and Sensitivity
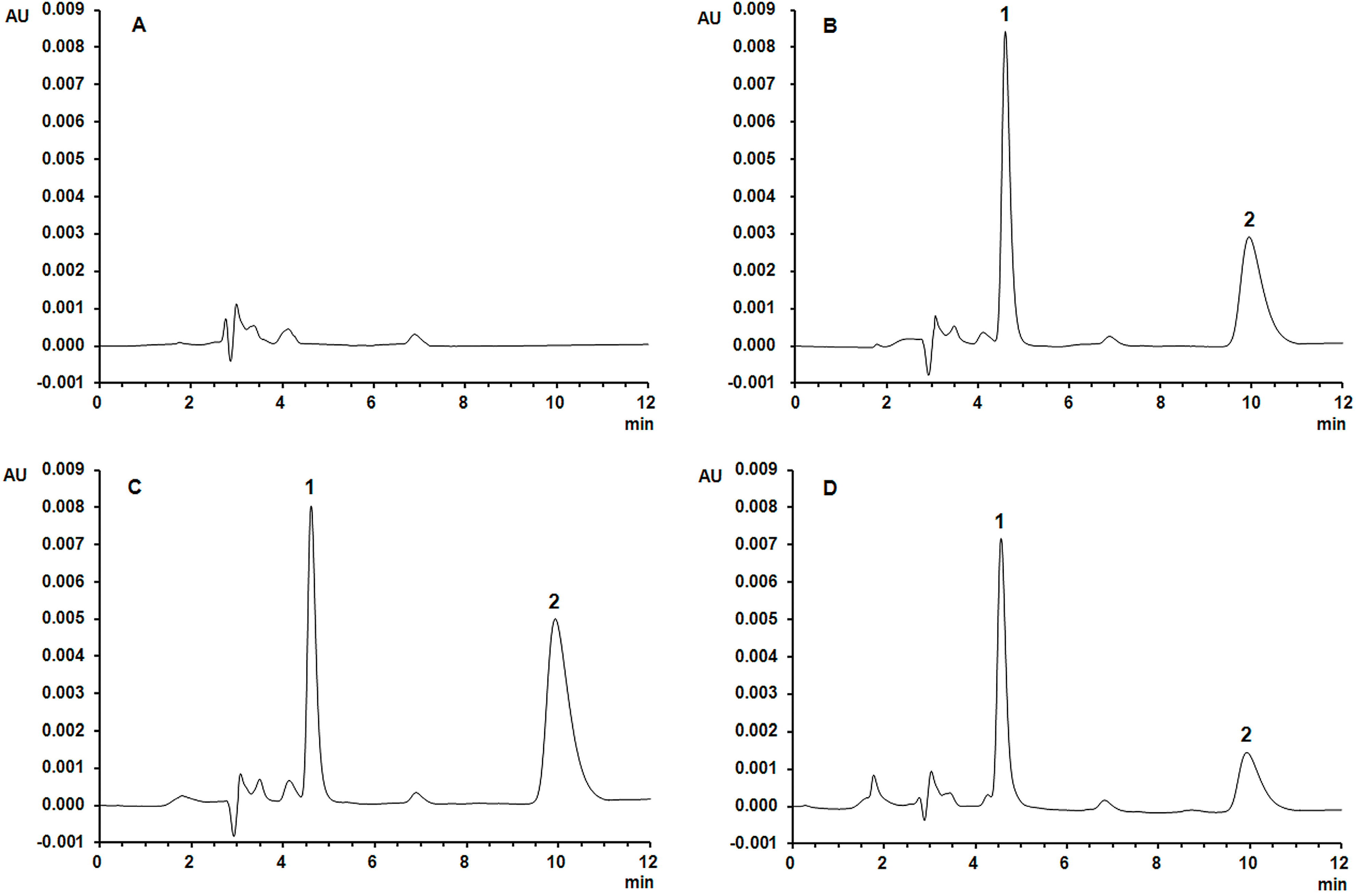
2.3. Precision and Accuracy
| Analyte | Concentration | Extraction Recovery (n = 3) | Matrix Effect (n = 3) | Intra-Day (n = 5) | Inter-Day (n = 5) | Accuracy (n = 5) | ||
|---|---|---|---|---|---|---|---|---|
| (μg/mL) | Mean ± SD (%) | Mean ± SD (%) | Mean ± SD (μg/mL) | RSD (%) | Mean ± SD (μg/mL) | RSD (%) | RE (%) | |
| Sinomenine | 0.25 | 79.3 ± 3.4 | 89.8 ± 4.1 | 0.268 ± 0.029 | 10.9 | 0.287 ± 0.025 | 8.6 | 7.2 |
| 4.00 | 78.5 ± 9.8 | 91.2 ± 2.8 | 4.16 ± 0.18 | 4.3 | 4.20 ± 0.23 | 5.5 | 3.3 | |
| 40.0 | 83.4 ± 4.2 | 93.5 ± 3.7 | 42.9 ± 1.9 | 4.4 | 41.9 ± 2.91 | 6.9 | 7.2 | |
2.4. Recovery, Matrix Effect and Stability
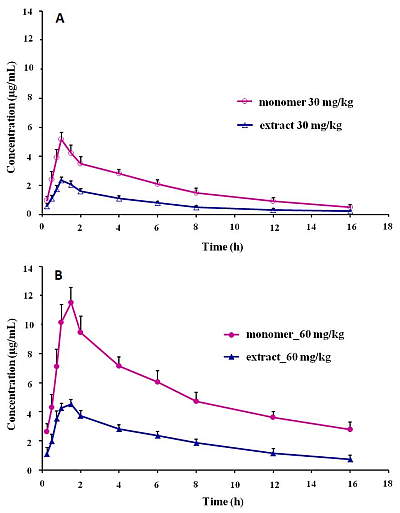
2.5. Pharmacokinetic Studies
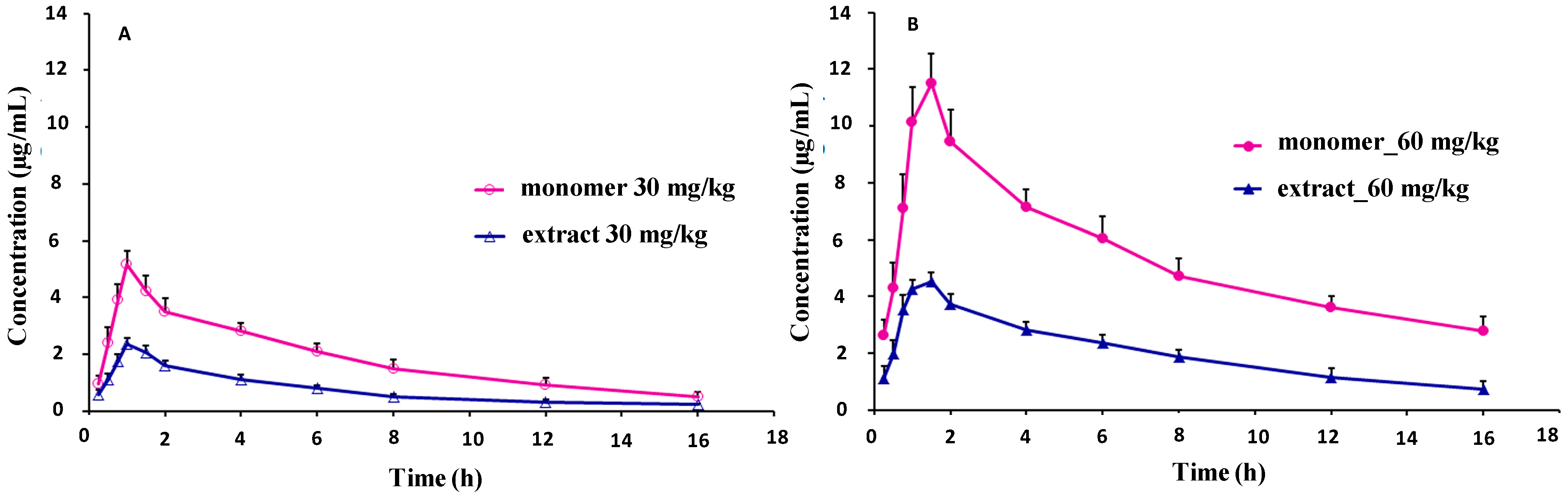
| Parameters | Sinomenine | Sinomenium acutum extract | ||
|---|---|---|---|---|
| 30 mg/kg | 60 mg/kg | 30 mg/kg | 60 mg/kg | |
| Tmax (h) | 1.083 ± 0.204 | 1.500 ± 0.316 | 1.083 ± 0.204 | 1.333 ± 0.258 |
| Cmax (μg/mL) | 5.235 ± 0.390 | 11.581 ± 0.942 | 2.397 ± 0.203 ** | 4.650 ± 0.186 ** |
| AUC0- t (mg·h/L) | 29.206 ± 4.062 | 78.879 ± 5.129 | 11.824 ± 0.690 ** | 32.205 ± 2.723 ** |
| AUC0–∞ (mg·h/L) | 32.823 ± 5.368 | 94.024 ± 11.248 | 13.310 ± 1.136 ** | 39.966 ± 8.740 ** |
| t1/2z (h) | 4.875 ± 0.635 | 5.767 ± 1.590 | 4.997 ± 1.314 | 6.442 ± 2.675 |
| CLz/F (L/kg/h) | 0.935 ± 0.152 | 0.646 ± 0.077 | 2.268 ± 0.195 ** | 1.553 ± 0.289 ** |
| Vz/F (L/h) | 6.491 ± 0.704 | 5.256 ± 1.021 | 16.145 ± 3.515 ** | 13.594 ± 2.458 ** |
| MRTinf (h) | 7.304 ± 0.889 | 8.801 ± 1.914 | 7.138 ± 1.416 | 9.582 ± 3.480 |
3. Experimental
3.1. Animals
3.2. Materials and Reagent
3.3. Apparatus and Chromatographic Conditions
3.4. Preparation of Sinomenium acutum Extract
3.5. Animal Experimental Design
3.6. Plasma Sample Preparation
3.7. Preparation of Calibration Standards and QC Samples
3.8. Method Validation
3.9. Pharmacokinetic and Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oravecz, M.; Mészáros, J. Traditional Chinese medicine: Theoretical background and its use in China. Orvosi Hetil 2012, 153, 723–731. [Google Scholar]
- Wang, Y.; Fan, X.; Qu, H.; Gao, X.; Cheng, Y. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr. Top. Med. Chem 2012, 12, 1356–1362. [Google Scholar]
- Xutian, S.; Cao, D.; Wozniak, J.; Junion, J.; Boisvert, J. Comprehension of the unique characteristics of traditional Chinese medicine. Am. J. Chin. Med 2012, 40, 231–244. [Google Scholar]
- Li, X.; Yang, G.; Zhang, Y.; Yang, J.; Chang, J.; Sun, X.; Zhou, X.; Guo, Y.; Xu, Y.; Liu, J. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in chinese. PLoS One 2013, 8, e60338. [Google Scholar]
- Zhao, X.X.; Peng, C.; Zhang, H.; Qin, L.P. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm. Biol 2012, 50, 1053–1061. [Google Scholar] [CrossRef]
- Jin, H.Z.; Wang, X.L.; Wang, H.B.; Wang, Y.B.; Lin, L.P.; Ding, J.; Qin, G.W. Morphinane alkaloid dimers from Sinomenium acutum. J. Nat. Prod 2008, 71, 127–129. [Google Scholar]
- Wang, X.L.; Liu, B.R.; Wang, J.R.; Chen, C.K.; Qin, G.W.; Lee, S.S. Two new morphinane alkaloids from Sinomenium acutum. J. Asian Nat. Prod. Res 2011, 13, 523–528. [Google Scholar]
- Cheng, J.J.; Tsai, T.H.; Lin, L.C. New alkaloids and cytotoxic principles from Sinomenium acutum. Planta Med 2012, 78, 1873–1877. [Google Scholar]
- Wang, X.L.; Liu, B.R.; Chen, C.K.; Qin, G.W.; Lee, S.S. Sinoscrewtine, an alkaloid with novel skeleton from the roots of Sinomenium acutum. Fitoterapia 2013, 84, 54–57. [Google Scholar]
- Zhao, Y.; Li, J.; Yu, K.; Liu, Y.; Chen, X. Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int. Immunopharmacol 2007, 7, 637–645. [Google Scholar]
- Zhou, H.; Wong, Y.F.; Wang, J.; Cai, X.; Liu, L. Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in rats. Biochem. Biophys. Res. Commun 2008, 376, 352–357. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.K. Immunosuppressive and anti-inflammatory activities of sinomenine. Int. Immunopharmacol 2011, 11, 373–376. [Google Scholar]
- Liu, L.; Resch, K.; Kaever, V. Inhibition of lymphocyte proliferation by the anti-arthritic drug sinomenine. Int. J. Immunopharmacol 1994, 16, 685–691. [Google Scholar]
- Ju, X.D.; Deng, M.; Ao, Y.F.; Yu, C.L.; Wang, J.Q.; Yu, J.K.; Cui, G.Q.; Hu, Y.L. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi 2010, 130, 1053–1060. [Google Scholar]
- Li, Y.F.; Wang, M.; Wang, X.Y.; Yu, H.S.; Kang, L.P.; Ma, B.P.; Ruan, J.X.; Zhang, Z.Q. Pharmacokinetic properties of albiflorin and paeoniflorin after oral administration of pure compound, Radix Paeoniae alba extract and danggui-shaoyao-san extract to rats. J. Asian Nat. Prod. Res 2011, 13, 117–127. [Google Scholar]
- Liu, J.Y.; Lee, K.F.; Sze, C.W.; Tong, Y.; Tang, S.C.; Ng, T.B.; Zhang, Y.B. Intestinal absorption and bioavailability of traditional Chinese medicines: A review of recent experimental progress and implication for quality control. J. Pharm. Pharmacol 2013, 65, 621–633. [Google Scholar]
- Pei, L.; Bao, Y.; Liu, S.; Zheng, J.; Chen, X. Material basis of Chinese herbal formulas explored by combining pharmacokinetics with network pharmacology. PLoS One 2013, 8, e57414. [Google Scholar]
- Qv, X.Y.; Jiang, J.G.; Piao, J.H. Pharmacodynamic studies of Chinese medicine at levels of whole animal, cell and molecular models. Curr. Med. Chem 2010, 17, 4521–4537. [Google Scholar]
- Zhu, Y.; Zhang, Z.; Zhang, M.; Mais, D.E.; Wang, M.W. High-throughput screening for bioactive components from traditional Chinese medicine. Comb. Chem. High Throughput Screen 2010, 13, 837–848. [Google Scholar]
- Li, Z.; Xu, C. The fundamental theory of traditional Chinese medicine and the consideration in its research strategy. Front. Med. 2011, 5, 208–211. [Google Scholar]
- Xu, M.; Wang, G.; Xie, H.; Huang, Q.; Wang, W.; Jia, Y. Pharmacokinetic comparisons of schizandrin after oral administration of schizandrin monomer, Fructus Schisandrae aqueous extract and Sheng-Mai-San to rats. J. Ethnopharmacol 2008, 115, 483–488. [Google Scholar]
- Mao, S.; Zhang, H.; Lv, L.; Zhu, Z.; Zhao, L.; Zhang, G.; Chai, Y. Rapid determination and pharmacokinetics study of lignans in rat plasma after oral administration of Schisandra chinensis extract and pure deoxyschisandrin. Biomed. Chromatogr 2011, 25, 808–815. [Google Scholar]
- Yang, S.; Zhang, K.; Lin, X.; Miao, Y.; Meng, L.; Chen, W.; Tang, X. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. J. Pharm. Biomed. Anal 2012, 67–68, 77–85. [Google Scholar]
- Long, L.H.; Wu, P.F.; Chen, X.L.; Zhang, Z.; Chen, Y.; Li, Y.Y.; Jin, Y.; Chen, J.G.; Wang, F. HPLC and LC-MS analysis of sinomenine and its application in pharmacokinetic studies in rats. Acta Pharmacol. Sin 2010, 31, 1508–1514. [Google Scholar]
- Liu, Z.Q.; Zhou, H.; Liu, L.; Jiang, Z.H.; Wong, Y.F.; Xie, Y.; Cai, X.; Xu, H.X.; Chan, K. Influence of co-administrated sinomenine on pharmacokinetic fate of paeoniflorin in unrestrained conscious rats. J. Ethnopharmacol 2005, 99, 61–67. [Google Scholar]
- Chen, W.; Zhou, Y.; Kang, J.; Li, Q.; Feng, X. Study on pharmacokinetics and absolute bioavailability of sinomenine in beagle dogs. Zhongguo Zhong Yao Za Zhi 2009, 34, 468–471. [Google Scholar]
- Yan, X.H.; Li, H.D.; Peng, W.X.; Liu, F.Q.; Shao, Y.; He, Y.Q. Determination of sinomenine HCl in serum and urine by HPLC and its pharmacokinetics in normal volunteers. Yao Xue Xue Bao 1997, 32, 620–624. [Google Scholar]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm. Drug Dispos 2011, 32, 427–445. [Google Scholar]
- Wu, H.; Zhu, Z.; Zhang, G.; Zhao, L.; Zhang, H.; Zhu, D.; Chai, Y. Comparative pharmacokinetic study of paeoniflorin after oral administration of pure paeoniflorin, extract of Cortex Moutan and Shuang-Dan prescription to rats. J. Ethnopharmacol 2009, 125, 444–449. [Google Scholar]
- Lu, T.; Song, J.; Huang, F.; Deng, Y.; Xie, L.; Wang, G.; Liu, X. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J. Ethnopharmacol 2007, 110, 412–418. [Google Scholar]
- Chan, K.; Liu, Z.Q.; Jiang, Z.H.; Zhou, H.; Wong, Y.F.; Xu, H.X.; Liu, L. The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J. Ethnopharmacol 2006, 103, 425–432. [Google Scholar]
- Liu, Z.Q.; Chan, K.; Zhou, H.; Jiang, Z.H.; Wong, Y.F.; Xu, H.X.; Liu, L. The pharmacokinetics and tissue distribution of sinomenine in rats and its protein binding ability in vitro. Life Sci 2005, 77, 3197–3209. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Z.H.; Zhou, H.; Ma, W.Z.; Wong, Y.F.; Liu, Z.Q.; Liu, L. The pharmacokinetic study of sinomenine, paeoniflorin and paeonol in rats after oral administration of a herbal product Qingfu Guanjiesu capsule by HPLC. Biomed. Chromatogr 2014, 2014. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Jiang, Z.H.; Chan, K.; Zhou, H.; Wong, Y.F.; Bian, Z.X.; Xu, H.X.; Liu, L. Pharmacokinetic interaction of paeoniflorin and sinomenine: Pharmacokinetic parameters and tissue distribution characteristics in rats and protein binding ability in vitro. J. Pharmacol. Sci 2005, 99, 381–391. [Google Scholar]
- Tsai, T.H.; Wu, J.W. Regulation of hepatobiliary excretion of sinomenine by P-glycoprotein in Sprague-Dawley rats. Life Sci 2003, 72, 2413–2426. [Google Scholar]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Chromatic properties and global phenolic composition by means of UV-Vis spectrophotometry. Food Chem 2013, 140, 217–224. [Google Scholar]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J.; Teixeira, N.; Mateus, N.; de Freitas, V. The phenolic chemistry and spectrochemistry of red sweet wine-making and oak-aging. Food Chem 2014, 152, 522–530. [Google Scholar]
- Samples Availability: Sample of the compound sinomenine is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, M.-F.; Zhao, Y.; Jiang, K.-Y.; Han, L.; Lu, X.-Y.; Wang, X.; Zuo, L.; Meng, S.-N. Comparative Pharmacokinetics Study of Sinomenine in Rats after Oral Administration of Sinomenine Monomer and Sinomenium Acutum Extract. Molecules 2014, 19, 12065-12077. https://doi.org/10.3390/molecules190812065
Zhang M-F, Zhao Y, Jiang K-Y, Han L, Lu X-Y, Wang X, Zuo L, Meng S-N. Comparative Pharmacokinetics Study of Sinomenine in Rats after Oral Administration of Sinomenine Monomer and Sinomenium Acutum Extract. Molecules. 2014; 19(8):12065-12077. https://doi.org/10.3390/molecules190812065
Chicago/Turabian StyleZhang, Mao-Fan, Yan Zhao, Kun-Yu Jiang, Long Han, Xiao-Yue Lu, Xin Wang, Lan Zuo, and Sheng-Nan Meng. 2014. "Comparative Pharmacokinetics Study of Sinomenine in Rats after Oral Administration of Sinomenine Monomer and Sinomenium Acutum Extract" Molecules 19, no. 8: 12065-12077. https://doi.org/10.3390/molecules190812065