Synthesis of Stabilized Myrrh-Capped Hydrocolloidal Magnetite Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of Capped Iron Oxide Nanoparticles
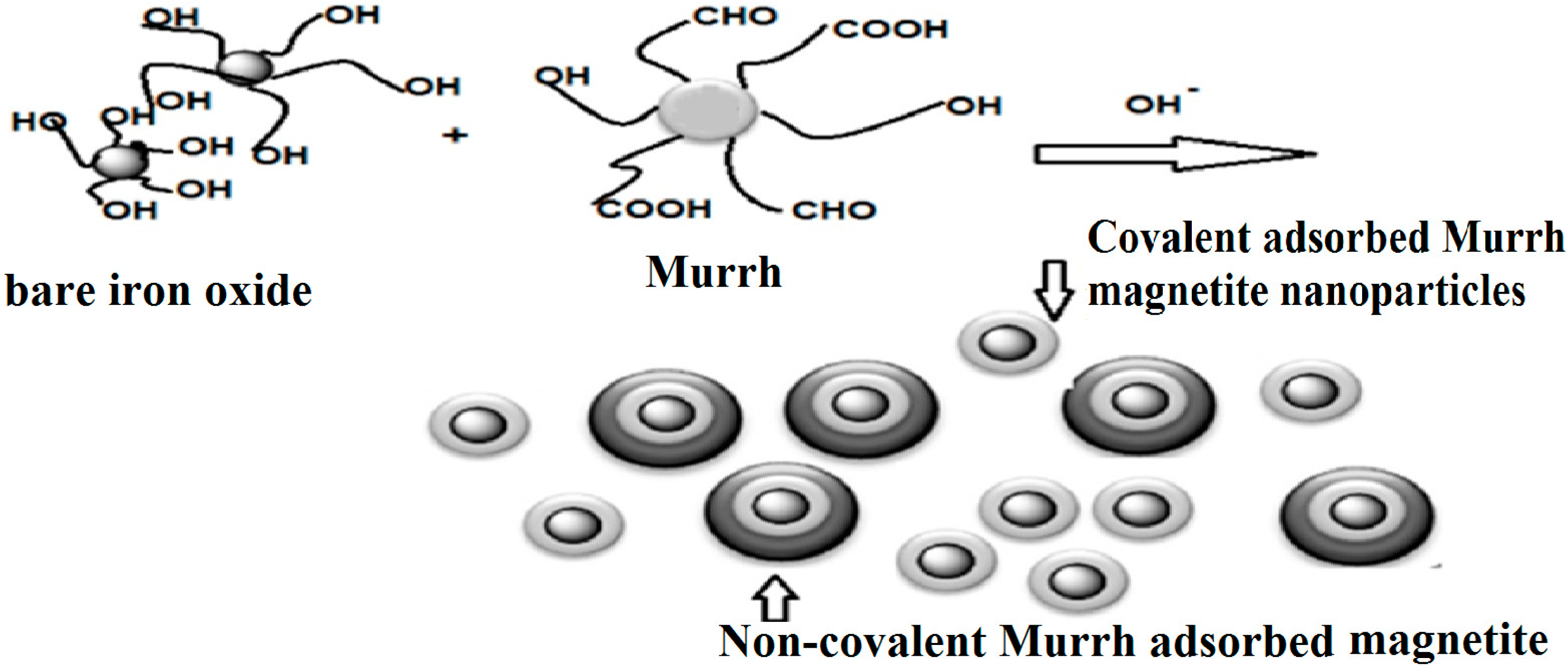
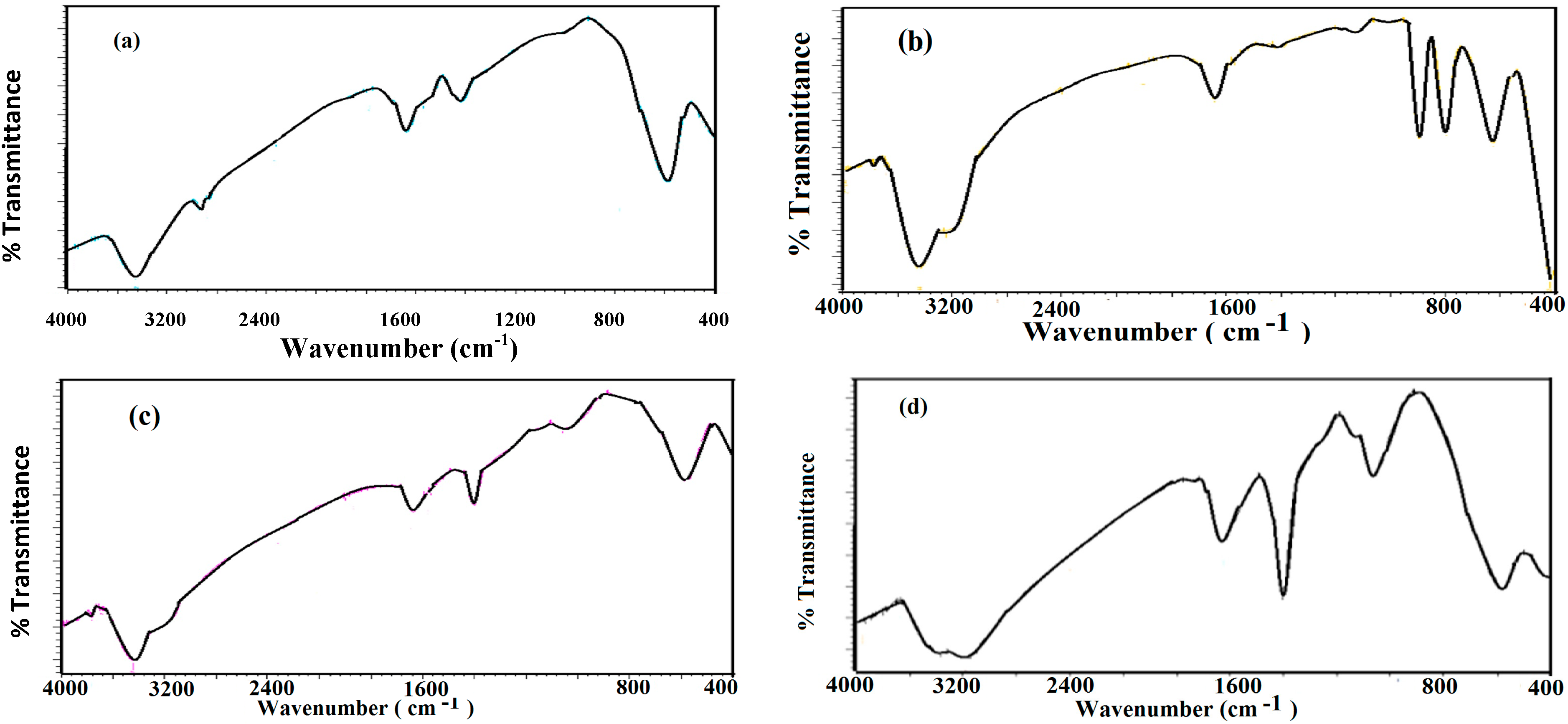
2.2. Characterization of Capped MNPs

| Standard Diffraction Data | Experimental Data of Fe3O4 | |
|---|---|---|
| Fe3O4 | γ-Fe2O3 | |
| 2θ | 2θ | 2θ |
| 30.1 | 30.28 | 29.99 |
| 35.43 | 35.69 | 35.51 |
| 43.06 | 43.35 | 43.23 |
| 53.41 | 53.87 | 53.59 |
| 56.96 | 57.42 | 57.12 |
| 62.53 | 63.03 | 62.81 |
| 73.97 | 74.56 | 74.27 |
2.3. Morphologies and Particle Size of the Prepared MNPs
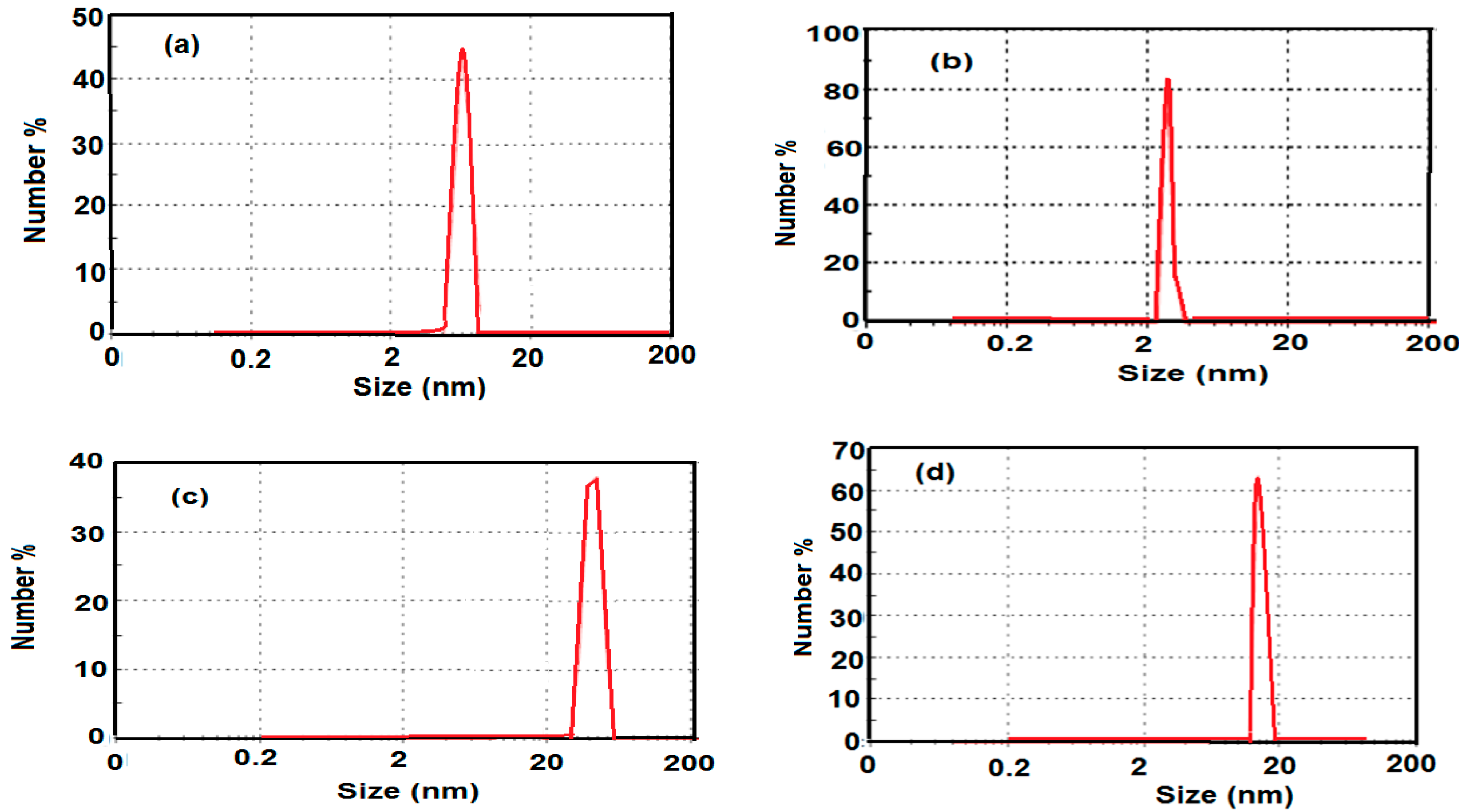
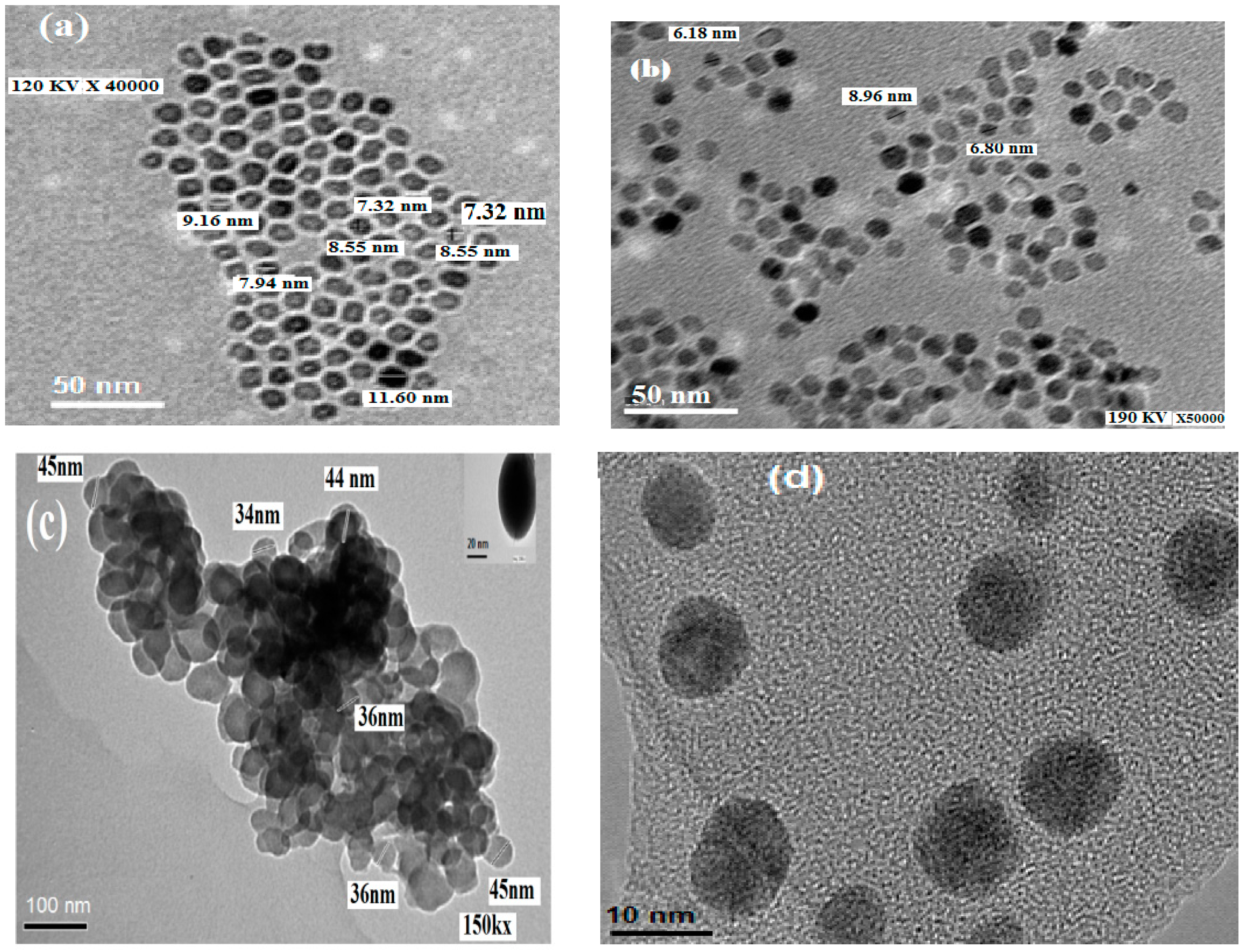
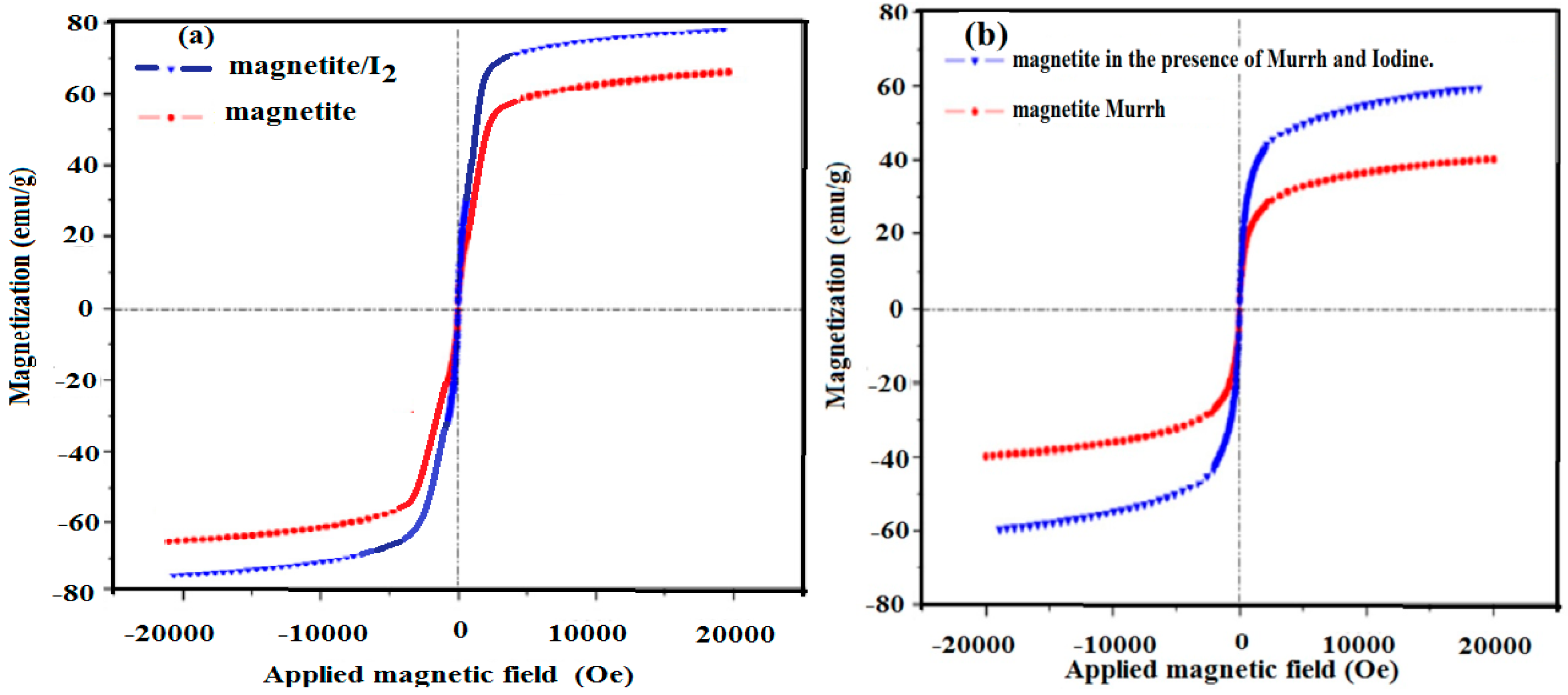
2.4. Magnetic Properties
3. Experimental Section
3.1. Materials
3.2. Preparation of Magnetite Nanoparticles
3.2.1. Preparation of Magnetite Nanoparticles (MNPs)
3.2.2. Preparation of Magnetite Capped with Myrrh
3.3. Characterization of Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, R.R.; Mather, J.G.; Kennaugh, J.H. Magnetic bones in human sinuses. Nature 1983, 301, 79–80. [Google Scholar]
- Basavaiah, K.V.; Rao, P.A. One-pot synthesis of superparamagnetic polyaniline microtubes and magnetite nanoparticles via self-assembly method. Curr. Nanosci. 2012, 8, 215–220. [Google Scholar] [CrossRef]
- Fidale, L.C.; Nikolajski, M.; Rudolph, T.; Dutz, S.; Schacher, F.H.; Heinze, T. Hybrid Fe3O4@amino cellulose nanoparticles in organic media—Heterogeneous ligands for atom transfer radical polymerizations. J. Colloid. Interface Sci. 2013, 390, 25–33. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4(M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Bakandritsos, A.; Georgakilas, V.; Tzitzios, V.; Petridis, D. Facile synthesis of capped γ-Fe2O3and Fe3O4nanoparticles. J. Mater Sci. 2006, 41, 5250–5256. [Google Scholar] [CrossRef]
- Yue, F.J.; Wang, S.; Lin, L.; Ding, H.F.; Wu, D. Effects of ferromagnet-molecule chemical bonding on spin injection in an Fe3O4-molecule granular system. J. Phys. D Appl. Phys. 2012, 45. [Google Scholar] [CrossRef]
- Unal, B.; Toprak, M.S.; Durmus, Z.; Sozeri, H.; Baykal, A. Synthesis, structural and conductivity characterization of alginic acid-Fe3O4 nanocomposite. J. Nanopart.Res. 2010, 8, 3039–3048. [Google Scholar]
- Chockalingam, A.M.; Babu, H.K.R.; Chittor, R.; Tiwari, J.P. Gum arabic modified Fe3O4 nanoparticles cross linked with collagen for isolation of bacteria. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef]
- Roque, A.C.A.; Bicho, A.; Batalha, I.L.; Cardoso, A.S.; Hussain, A. Biocompatible and bioactive gum Arabic coated iron oxide magnetic nanoparticles. J.Biotechnol. 2009, 144, 313–320. [Google Scholar] [CrossRef]
- Kannan, R.; Rahing, V.; Cutler, C.; Pandrapragada, R.; Katti, K.K.; Kattumuri, V.; Robertson, J.D.; Casteel, S.J.; Jurisson, S.; Smith, C.; et al. Nanocompatible chemistry toward fabrication of target-specific gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 11342–11343. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Native-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett. 2002, 2, 25–28. [Google Scholar] [CrossRef]
- Park, C.; Lim, K.H.; Kwon, D.; Yoon, T.H. Biocompatible quantum dot nanocolloids stabilized by gum Arabic. Bull. Korean Chem. Soc. 2008, 29, 1277–1279. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. A general strategy for nanocrystals synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef]
- Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef]
- Fried, T.; Shemer, G.; Markovich, G. Ordered Two-dimensional arrays of ferrite nanoparticles. Adv. Mater. 2001, 13, 1158–1161. [Google Scholar] [CrossRef]
- Yu, M.K.; Jeong, Y.Y.; Park, J.; Park, S.; Kim, J.W.; Min, J.J.; Kim, K.; Jon, S. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew. Chem. Int. Ed. 2008, 47, 5362–5365. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.; Lee, S.; Khim, Z.G.; Char, K.; Hyeon, T. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. J. Am. Chem. Soc. 2000, 122, 8581–8582. [Google Scholar] [CrossRef]
- Beketov, I.V.; Safronov, A.P.; Medvedev, A.I.; Alonso, J.; Kurlyandskaya, G.V.; Bhagat, S.M. Iron oxide nanoparticles fabricated by electric explosion of wire: Focus on magnetic nanofluids. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Komogortsev, S.V.; Kurlyandskaya, G.V.; Medvedev, A.I.; Leiman, D.V.; Larrañaga, A.; Bhagat, S.M. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in Cancer Medicine. Phys. Today 2012, 65. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Atta, A.M.; Dyab, A.K.F. Coated Magnetite Nanoparticles, Method for the Preparation thereof and Their Use. EP 13167616.5, 18 March 2013. [Google Scholar]
- Khalil, M.I. Process for Preparing Magnetic (Fe3O4) and Derivatives thereof. EP 2505558 A1, 16 January 2013. [Google Scholar]
- Jolivet, J.P.; Chaneac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 5, 481–487. [Google Scholar] [CrossRef]
- Cornel, R.M.; Schwertmann, U. The Iron Oxides, Structure, Properties, Reactions and Uses; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Madrakian, T.; Afkhami, A.; Zolfigo, M.A.; Ahmadi, M.; Koukabi, N. Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples. Nano-Micro Lett. 2012, 4, 57–63. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed.; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Nagata, T.; Fukushi, K. Prediction of iodate adsorption and surface speciation on oxides by surface complexation modeling. Geochim. Cosmochim. Acta 2010, 74, 6000–6013. [Google Scholar] [CrossRef]
- Hanuša, L.O.; Řezankab, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh–Commiphora Chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2005, 149, 3–28. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Thanha, A.; Luke, A.W. Green Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; Gonzales-Carreno, T.; Serna, J.C. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36. [Google Scholar] [CrossRef]
- Morales, M.P.; Pecharroman, C.; Gonzales-Carreno, T. Structural characteristics of uniform γ-Fe2O3particles with different axial (length/width) ratios. J. Solid StateChem. 1994, 108, 158–163. [Google Scholar] [CrossRef]
- Belin, T.; Guigue-Millot, N.; Caillot, T.; Aymes, D.; Niepce, J.C. Influence of grain size, oxygen stoichiometry, and synthesis conditions on the γ-Fe2O3 vacancies ordering and lattice parameters. J. Solid State Chem. 2002, 163, 459–465. [Google Scholar] [CrossRef]
- Morales, M.P.; Andres-Verges, M.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J. Structural effects on the magnetic properties of γ-Fe2O3 nanoparticles. J. Magn. Magn. Mater. 1999, 203, 146–148. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.I.; Martinez, B.; Sandiumenge, F. Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Daou, T.J.; Greneche, J.M.; Pourroy, G.; Buathong, S.; Derory, A.; Ulhaq-Bouillet, C.; Donnio, B.; Guillon, D.; Begin-Colin, S. Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chem. Mater. 2008, 20, 5869–5875. [Google Scholar] [CrossRef]
- Shaswant, B.S.; Dong-Hwang, C. Fast removal of copper ions by gum Arabic modified nano-adsobent. J. Hazard. Mater. 2007, 147, 792–799. [Google Scholar] [CrossRef]
- Stolnik, S.; Illum, L.; Davis, S.S. Long circulating microparticulate drug carriers. Adv. Drug Deliver. Rev. 1995, 16, 195–214. [Google Scholar] [CrossRef]
- Feitknecht, W.; Mannweiler, U. Der mechanismus der umwandlung von γ-zu-α-eisensesquioxid. Helv.Chim. Acta 1967, 50, 570–581. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgensrahlen. Available online: http://www.sajs.co.za/sites/default/files/publications/pdf/Kroon_Sci%20Corr.pdf (accessed on 21 July 2014).
- Wan, J.; Cai, W.; Meng, X.; Liu, E. Monodisperse water-soluble magnetite nanoparticles prepared by polyol process for high-performance magnetic resonance imaging. Chem. Commun. 2007, 47, 5004–5006. [Google Scholar]
- Zhao, X.; Shi, Y.; Wang, T.; Cai, Y.; Jiang, G. Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J. Chromatogr. A 2008, 1188, 140–147. [Google Scholar] [CrossRef]
- Tan, C.J.; Tong, Y.W. Preparation of superparamagnetic ribonuclease A surface-imprinted submicrometer particles for protein recognition in aqueous media. Anal. Chem. 2007, 79, 299–306. [Google Scholar] [CrossRef]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of oxidation on the magnetization of nanoparticulate magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Burke, N.A.D.; Stöver, H.D.H.; Dawson, F.P. Magnetic nanocomposites: Preparation and characterization of polymer-coated iron nanoparticles. Chem. Mater. 2002, 14, 4752–4761. [Google Scholar] [CrossRef]
- Vellora, V.; Padil, T.; Rouha, M.; Herník, M. Hydrocolloid-stabilized magnetite for efficient removal of radioactive phosphates. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Trudel, S.; Jones, C.H.W.; Hill, R.H. Magnetic properties of nanocrystallineiron oxide/amorphous manganese oxide nanocomposite thin films preparedviaphotochemical metal-organic deposition. J. Mater. Chem. 2007, 17, 2206–2218. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Serna, C.J. Magnetic properties of uniform γ-Fe2O3nanoparticles smaller than 5nm prepared by laser pyrolysis. J. Mater. Res. 1999, 14, 3066–3072. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds magnetite nanoparticles are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Atta, A.M.; Al-Lohedan, H.A.; Al-Hussain, S.A. Synthesis of Stabilized Myrrh-Capped Hydrocolloidal Magnetite Nanoparticles. Molecules 2014, 19, 11263-11278. https://doi.org/10.3390/molecules190811263
Atta AM, Al-Lohedan HA, Al-Hussain SA. Synthesis of Stabilized Myrrh-Capped Hydrocolloidal Magnetite Nanoparticles. Molecules. 2014; 19(8):11263-11278. https://doi.org/10.3390/molecules190811263
Chicago/Turabian StyleAtta, Ayman M., Hamad A. Al-Lohedan, and Sami A. Al-Hussain. 2014. "Synthesis of Stabilized Myrrh-Capped Hydrocolloidal Magnetite Nanoparticles" Molecules 19, no. 8: 11263-11278. https://doi.org/10.3390/molecules190811263





