Fluorescent Probes for Exploring Plant Cell Wall Deconstruction: A Review
Abstract
:1. Introduction
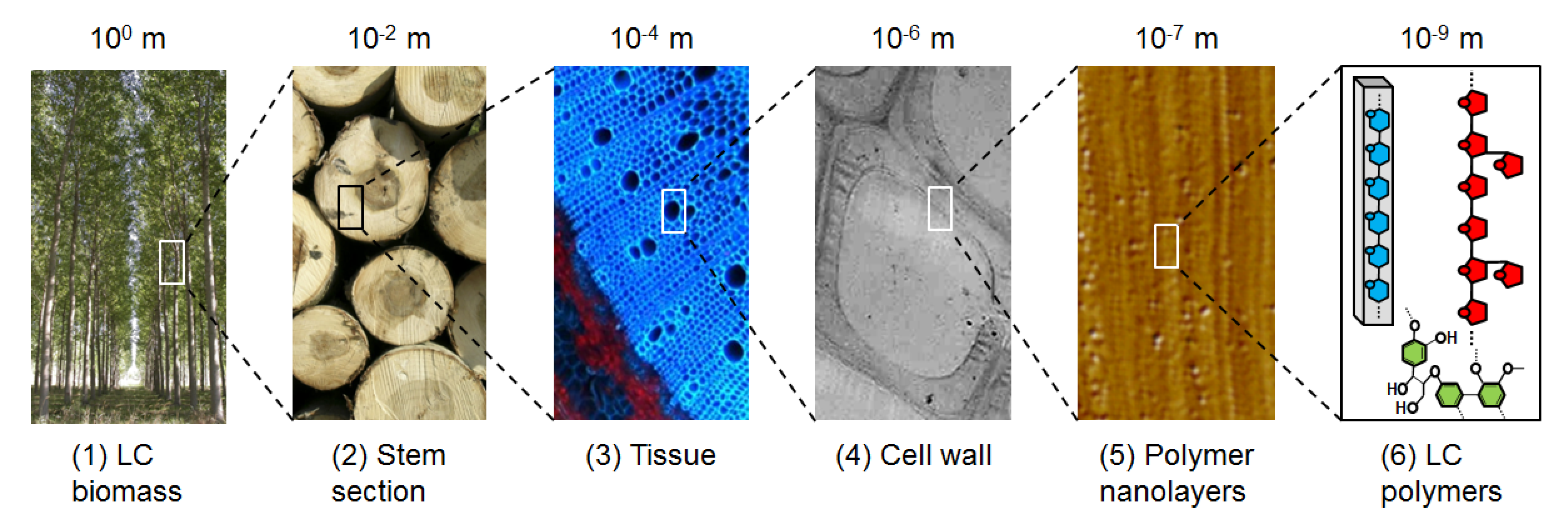
- -
- More efficient enzymes: engineering existing enzymes and mining natural diversity of living organisms degrading LC, to create the most efficient biocatalyst cocktail;
- -
2. Fluorophores: Variety and Properties
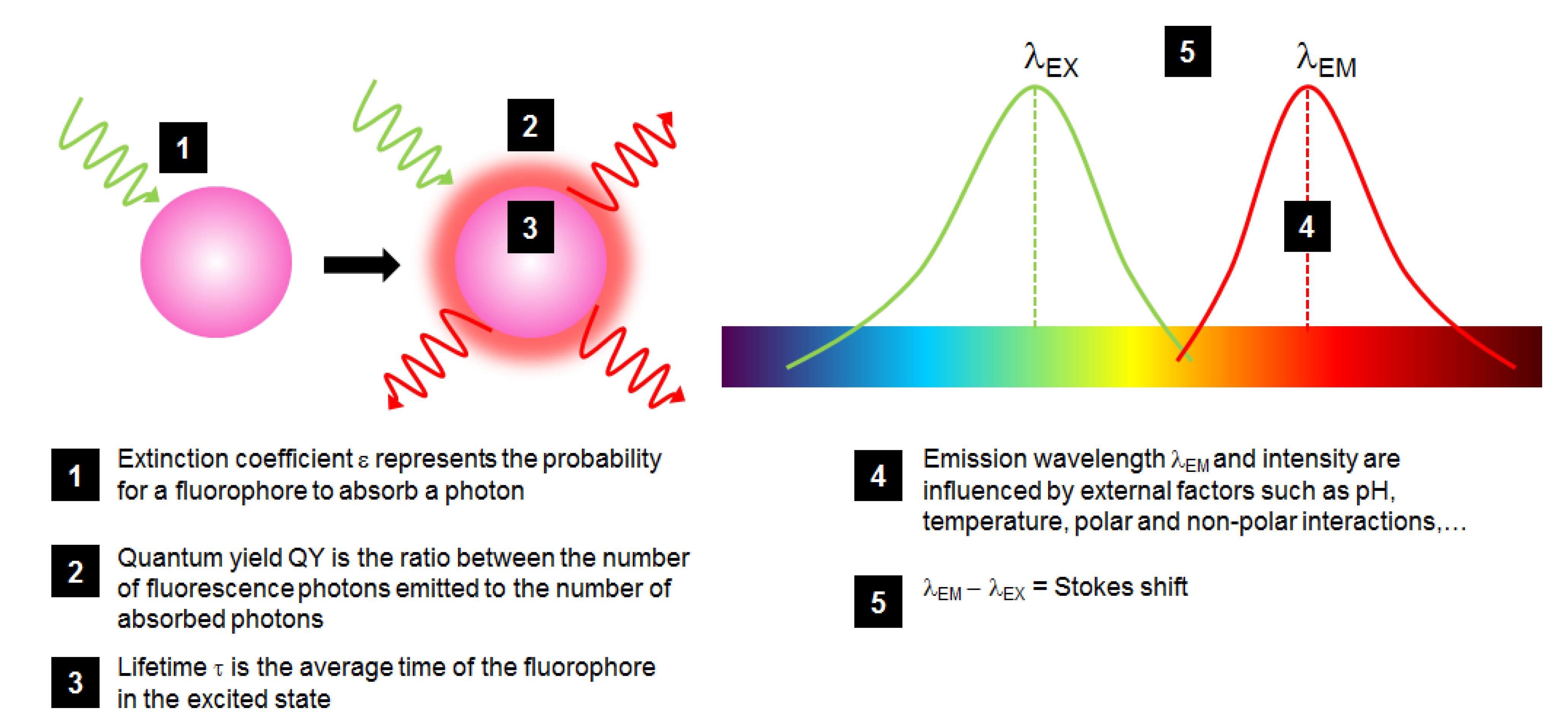
2.1. Definitions of Fluorophores
- -
- The probability for a fluorophore to absorb a photon is called the extinction coefficient ε, which is at a maximum at λEX;
- -
- The ratio between the number of fluorescence photons emitted to the number of absorbed photons is called quantum yield (QY or φ, comprised between 0 and 1), describing how efficient the fluorescence process is;
- -
- The brightness of a fluorophore is defined by ε × QY which is a good indicator to compare different fluorophores;
- -
- Fluorescence lifetime τ is the average duration in which the fluorophore remains in the excited state: when it returns to the ground state, it follows an exponential decay, in the order of ns. The less long this time is, the better the fluorophore sensitivity is. Lifetime is highly dependent on fluorophore environment and interaction;
- -
- The λEX – λEM difference is called the Stokes shift: a large one is preferred to distinguish between excitation and emission spectra, a critical measurement of the overlapping of fluorophore excitation and emission spectra for accurate and sensitive analysis.
- -
- Spectral properties of the fluorophore: excitation and emission maximum wavelengths and bandwidths, brightness, photostability;
- -
- Chemical properties: solubility, stability, ability to be conjugated to another biomolecule;
- -
- Physical properties: molecular weight, size, oligomerisation properties.
2.2. Small Organic Fluorophores
| Fluorescein | Tetramethylrhodamine | Alexa Fluor® 488 | GFP | QD (CdSe/ZnS) | |
|---|---|---|---|---|---|
| Maximum Excitation/Emission Wavelengths (nm) | 490/520 | 544/573 | 495/519 | 395/504 | 405/605 |
| QY | 0.90 | 0.28 | 0.92 | 0.79 | 0.40 |
| ε (M−1·cm−1) | 80,000 | 99,000 | 73,000 | 30,000 | 2,400,000 |
| Brightness | 72,000 | 27,700 | 67,000 | 21,600 | 1,000,000 |
| Fluorescence Lifetime (ns) | 4.1 | 1.6 | 4.1 | 2.7 and 3.3 | >10 |
| Hydrodynamic radius (nm) | 0.4 | 0.4 | 0.4 | 1.7 | 10 |
| Molecular Weight (Da) | 389 | 444 | 643 | ~28,000 | N/A |
2.3. Fluorescent Proteins
2.4. Inorganic Fluorophores
2.5. Fluorophore Imaging
2.5.1. Sample Preparation
2.5.2. Microscopes
- -
- A wide-field microscope for thin samples, but axial resolution is limited;
- -
- A confocal laser scanning microscope (CLSM) for thicker samples (100 µm and below) since this instrument can image sections of samples in the axial direction with a resolution depending on the excitation wavelength λEX and the objective NA (theoretical axial resolution is 1.4λ/NA2);
- -
- A multiphoton microscope. In order to increase spatial resolution, a two-photon absorption technique can be set up. Indeed, a fluorophrore can be equivalently excited by a photon with a wavelength λEX or by two photons with a wavelength of approximately 2λEX. As a result, the excitation volume of the fluorophore is limited to the focal point and is not spread. Since the excitation wavelength is doubled, it is twice less energetic: this technique limits sample autofluorescence and is much less damaging. Moreover, longer wavelengths are less absorbed by the sample and are also less affected by light scattering, allowing a better penetration in the sample. It is also particularly convenient when fluorophore observation requires UV excitation because two-photon infra-red light is enough to image sample, limiting sample and fluorophore damaging (for a review on multiphoton microscopy see [22]). However, multiphoton microscopy necessitates a femtosecond pulsed laser, which is a very expensive instrument.
2.5.3. Microscopy Imaging Techniques
- -
- For mapping fluorescent probes in samples, simple imaging techniques are routinely applied;
- -
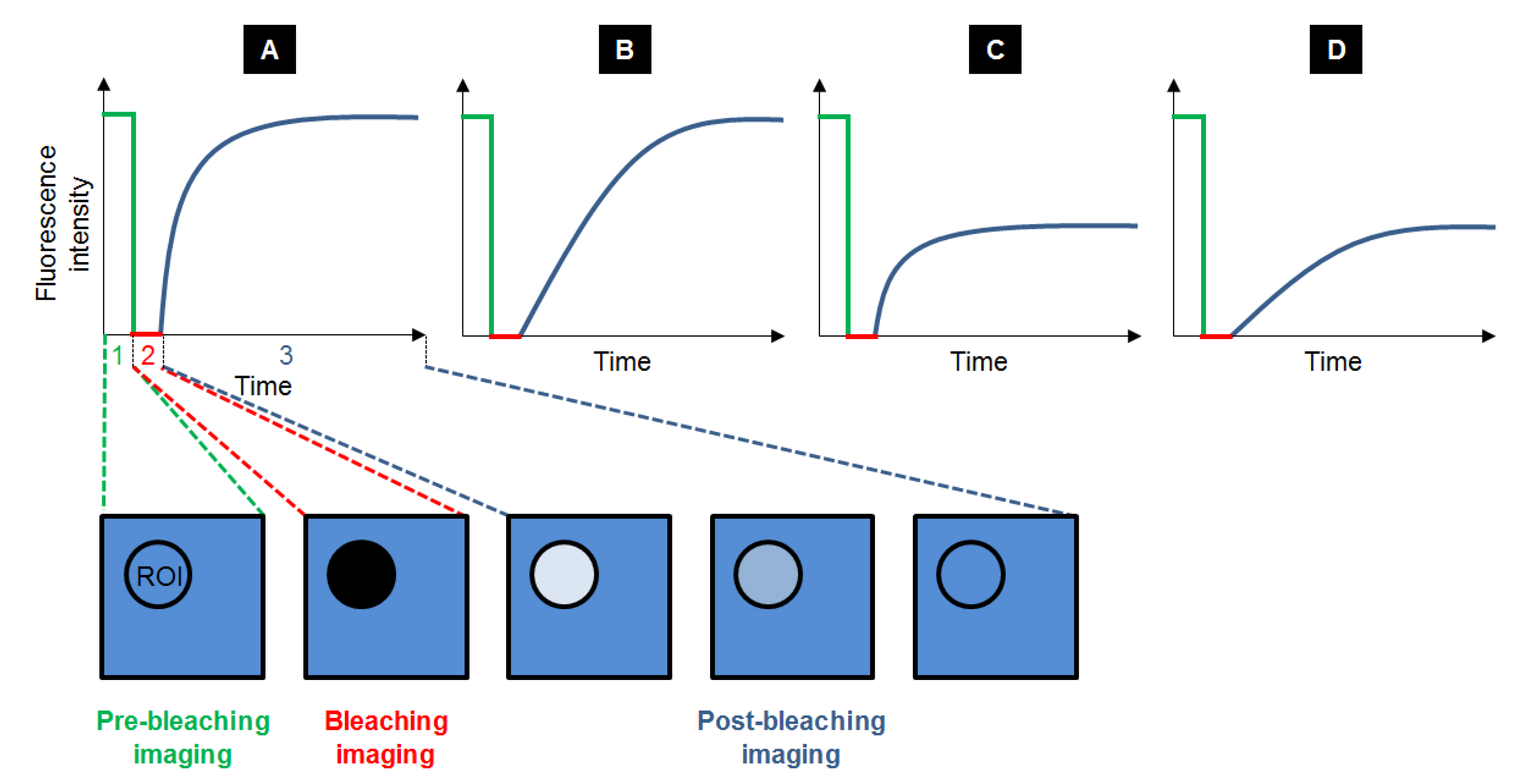
- For determining the diffusion and interaction processes of fluorescent probes, photobleaching techniques are very useful. They are performed in a CLSM, using a fast scan imaging (compromise between spatial and temporal information) to minimize laser intensity during imaging (except for the bleaching step);
- -
- For evaluating some interaction processes at molecular scale, measuring the energy transfer between the fluorescent probe and another endogenous fluorophore from the PCW is valuable but difficult to set up correctly.
- -
- The mobile and immobile fractions (MF and IF);
- -
- The half-life time τ1/2, which is the time needed to reach half of the maximum/final recovery intensity. Using Equation (1) [24], where r is the radius of the bleached ROI, gives access to the diffusion constant D:



3. Fluorescent Probe Families
3.1. Definition
- -
- Covalent labelling: a covalent bond is created between the fluorophore and the biomolecule. Most common reactions are between the isothiocyanate function in activated fluorophores such as fluorescein and amine functions of amino acid residues in proteins which can lead to the creation of a covalent bond at pH 9.0 or above. Functionalized QDs with carboxylate or amine functions can conjugate to primary amines or thiol functions;
- -
- Coordination labelling: a coordination between poly-histidine motifs in proteins can strongly conjugate to metals (Co, Cu, Fe, Mn, Ni, Zn) such as those found in QDs;
- -
- Heterologous expression: in the case of a recombinant biomolecule like protein, the gene sequence coding for a fluorescent protein can be added before or after that of the biomolecule, so that the two molecules are translated on the same polypeptide.
- -
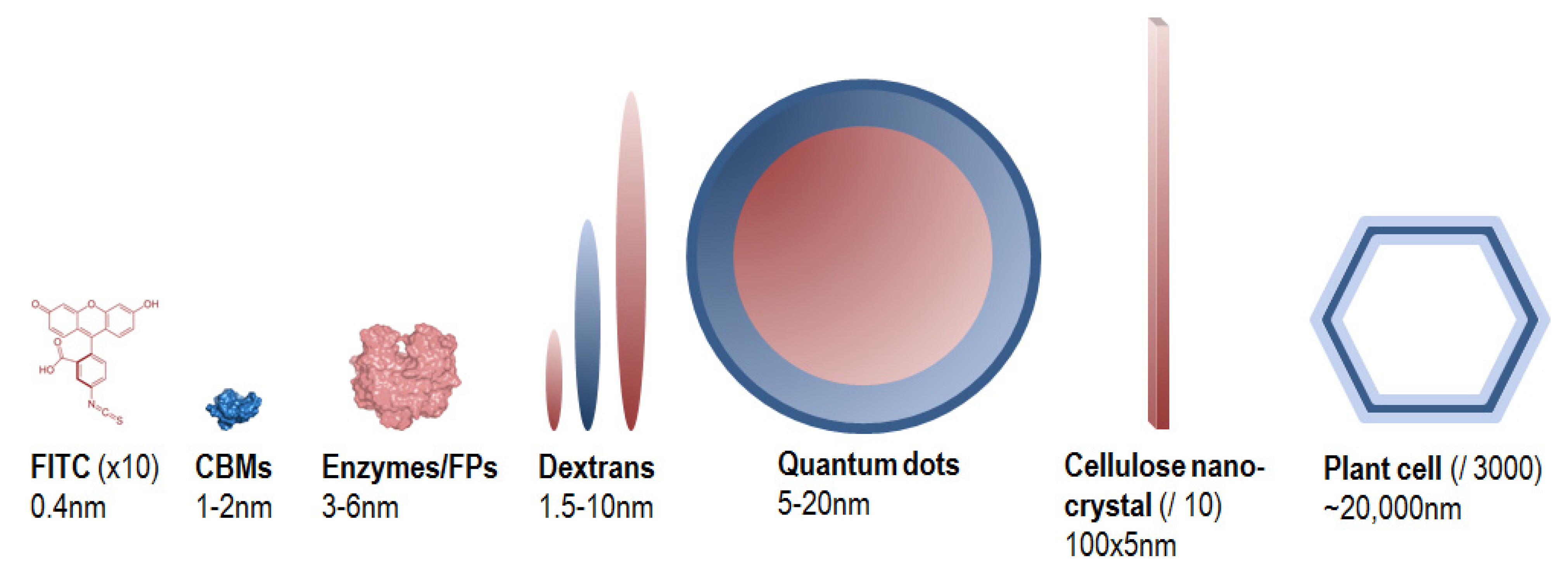
- During the conjugation reaction, the activated fluorophore may react with different chemical sites on the biomolecule, unless there is only one available, which is quite rare;
- -
- Even if a gel-filtration or dialysis of the conjugating reaction mixture is performed to purify the reaction product, the size difference between the biomolecule and the biomolecule/fluorophore conjugate can be very small (it is particularly true when the biomolecule is much larger than the fluorophore).
3.2. Small Fluorescent Probes and QDs
- -
- Calcofluor white binds cellulose but not with a high specificity compared to other plant polymers [34];
- -
- Congo red binds more strongly xyloglucan compared to cellulose but also binds proteins [34];
- -
- Phloroglucinol stains lignin in red proportionally to the lignin content;
- -
- Safranine binds lignified tissues in plants and wood, so its red fluorescence is a relevant sensor for differentiating cellulose-rich and lignin-rich cell walls [35];
| Small Fluorescent Molecules | Fluorescent Proteins | Quantum Dots | ||
|---|---|---|---|---|
| Spectral properties | Brightness | Low to medium | Medium | High |
| Photostability | Low, prone to photobleaching | Low, prone to photobleaching | High | |
| Stokes shift | Narrow | Narrow | Large | |
| Lifetime | Long | Long | Short | |
| Available at various excitation/emission λ | Yes | Yes | Yes | |
| Physical properties | Size (hydrodynamic diameter) | Small <1 nm | Medium >3 nm Prone to oligomerization | Large >10 nm |
| Chemical properties | Preparation | Commercial or lab dyes | Commercial genes | Commercial or lab |
| Labelling | Several derivatives can react with many functions DoL can be difficult to determine | Recombinant cloning DoL is known by construction | Difficult to control with accuracy | |
| Conjugate stability | High | Medium | Weak | |
| Other | Hydrophilic or hydrophobic Generic small fluorophores are cheap | Blinking (decreases QY), possibly toxic |

3.3. Polymers as Fluorescent Probes
3.4. Proteins as Fluorescent Probes
3.4.1. Proteins with no Catalytic Activity: CBMs
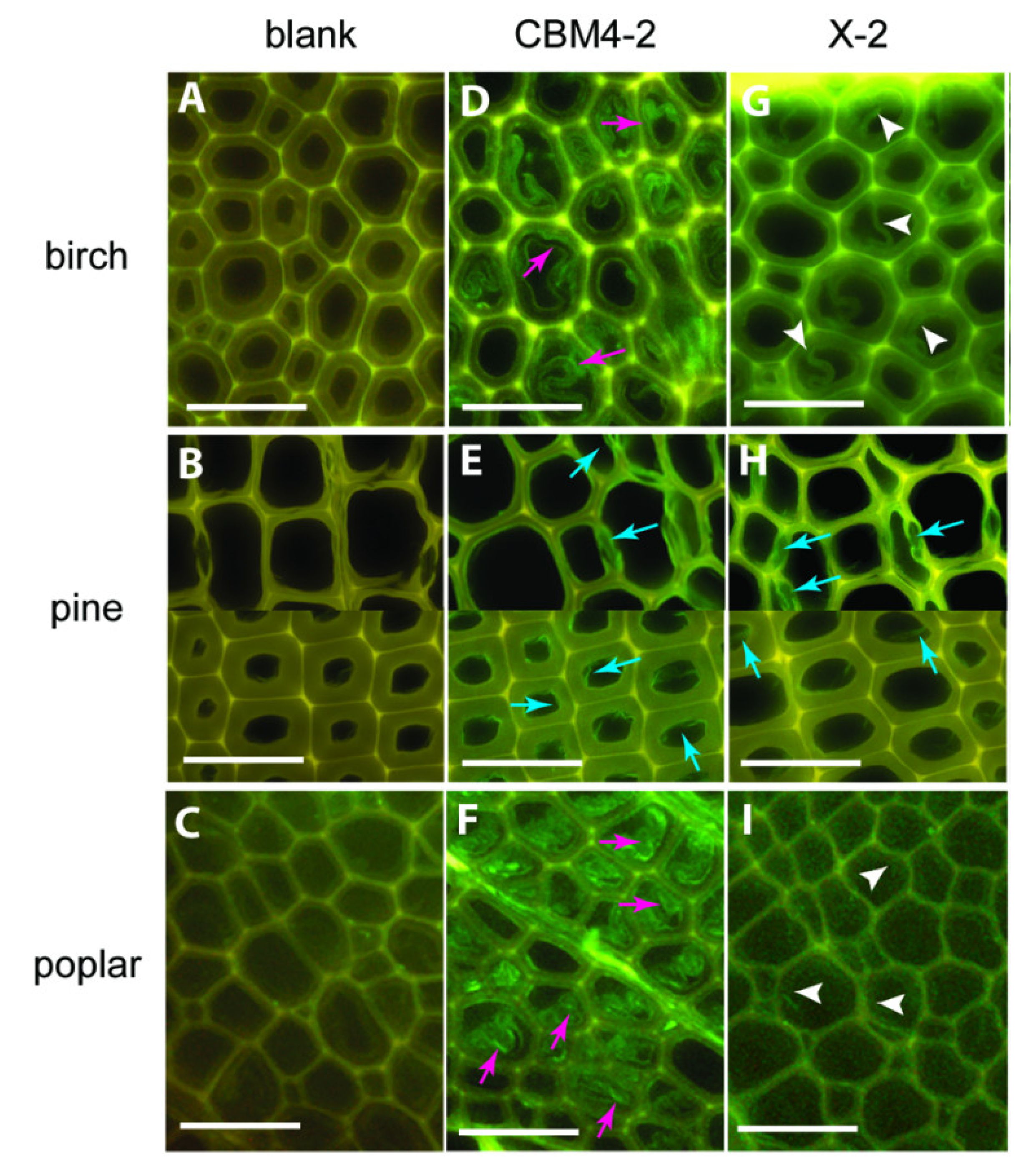
3.4.2. Proteins with Catalytic Activity: Enzymes
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Zhao, X.B.; Zhang, L.H.; Liu, D.H. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel. Bioprod. Bior. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Inouye, H.; Zhang, Y.; Yang, L.; Venugopalan, N.; Fischetti, R.F.; Gleber, S.C.; Vogt, S.; Fowle, W.; Makowski, B.; Tucker, M.; et al. Multiscale deconstruction of molecular architecture in corn stover. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.N.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Zhao, X.B.; Zhang, L.H.; Liu, D.H. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuel. Bioprod. Bior. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Mortimer, J.C.; Miles, G.P.; Brown, D.M.; Zhang, Z.N.; Segura, M.P.; Weimar, T.; Yu, X.L.; Seffen, K.A.; Stephens, E.; Turner, S.R.; et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 17409–17414. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Simmons, B.A.; Henry, R.J. Assessment of lignocellulosic biomass using analytical spectroscopy: An evolution to high-throughput techniques. Bioener. Res. 2014, 7, 1–23. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.M.; Tesso, T.; Dowell, F.; Wang, D.H. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energ. 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.C.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef]
- Tetard, L.; Passian, A.; Farahi, R.H.; Kalluri, U.C.; Davison, B.H.; Thundat, T. Spectroscopy and atomic force microscopy of biomass. Ultramicroscopy 2010, 110, 701–707. [Google Scholar] [CrossRef]
- Opitz, B.; Prediger, A.; Luder, C.; Eckstein, M.; Hilterhaus, L.; Lindner, P.; Beutel, S.; Scheper, T.; Liese, A. In situ microscopy for in-line monitoring of the enzymatic hydrolysis of cellulose. Anal. Chem. 2013, 85, 8121–8126. [Google Scholar] [CrossRef]
- Coletta, V.C.; Rezende, C.A.; da Conceiçãao, F.R.; Polikarpov, I.; Guimarães, F.E.G. Mapping the lignin distribution in pretreated sugarcane bagasse by confocal and fluorescence lifetime imaging microscopy. Biotech. Biofuels 2013, 6, 43. [Google Scholar]
- Donaldson, L.A.; Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. J. Microsc. 2013, 251, 178–187. [Google Scholar] [CrossRef]
- DeMartini, J.D.; Pattathil, S.; Avci, U.; Szekalski, K.; Mazumder, K.; Hahn, M.G.; Wyman, C.E. Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ. Sci. 2011, 4, 4332–4339. [Google Scholar] [CrossRef]
- Hötzer, B.; Medintz, I.L.; Hildebrandt, N. Fluorescence in nanobiotechnology: Sophisticated fluorophores for novel applications. Small 2012, 8, 2297–2326. [Google Scholar] [CrossRef]
- Sjoback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta A 1995, 51, L7–L21. [Google Scholar]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Pakhomov, A.A.; Martynov, V.I. GFP family: Structural insights into spectral tuning. Chem. Biol. 2008, 15, 755–764. [Google Scholar] [CrossRef]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Billinton, N.; Knight, A.W. Seeing the wood through the trees: A review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Anal. Biochem. 2001, 291, 175–197. [Google Scholar] [CrossRef]
- Ustione, A.; Piston, D.W. A simple introduction to multiphoton microscopy. J. Microsc. 2011, 243, 221–226. [Google Scholar] [CrossRef]
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell. Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef]
- Sprague, B.L.; Pego, R.L.; Stavreva, D.A.; McNally, J.G. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 2004, 86, 3473–3495. [Google Scholar] [CrossRef]
- Sprague, B.L.; McNally, J.G. FRAP analysis of binding: Proper and fitting. Trends Cell Biol. 2005, 15, 84–91. [Google Scholar] [CrossRef]
- Mueller, F.; Karpova, T.S.; Mazza, D.; McNally, J.G. Monitoring dynamic binding of chromatin proteins in vivo by fluorescence recovery after photobleaching chromatin remodeling. In Methods in Molecular Biology; Morse, R.H.H., Ed.; Humana Press: New York, NY, USA, 2012; Volume 833, pp. 153–176. [Google Scholar]
- Day, R.N.; Davidson, M.W. Fluorescent proteins for FRET microscopy: Monitoring protein interactions in living cells. Bioessays 2012, 34, 341–350. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P.C. Advanced fluorescence microscopy techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Snapp, E.; Kenworthy, A. Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Biol. 2001, 2, 444–456. [Google Scholar] [CrossRef]
- Fricker, M.; Runions, J.; Moore, I. Quantitative fluorescence microscopy: From art to science. Annu. Rev. Plant. Physiol. 2006, 57, 79–107. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: Oxford, UK, 2008. [Google Scholar]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef]
- Bond, J.; Donaldson, L.; Hill, S.; Hitchcock, K. Safranine fluorescent staining of wood cell walls. Biotech. Histochem. 2008, 83, 161–171. [Google Scholar]
- Thomas, J.; Ingerfeld, M.; Nair, H.; Chauhan, S.; Collings, D. Pontamine fast scarlet 4B: A new fluorescent dye for visualising cell wall organisation in radiata pine tracheids. Wood Sci Technol 2013, 47, 59–75. [Google Scholar]
- Vissenberg, K.; Martinez-Vilchez, I.M.; Verbelen, J.-P.; Miller, J.G.; Fry, S.C. In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell 2000, 12, 1229–1237. [Google Scholar] [CrossRef]
- Tobimatsu, Y.; Wagner, A.; Donaldson, L.; Mitra, P.; Niculaes, C.; Dima, O.; Kim, J.I.; Anderson, N.; Loque, D.; Boerjan, W.; et al. Visualization of plant cell wall lignification using fluorescence-tagged monolignols. Plant J. 2013, 76, 357–366. [Google Scholar] [CrossRef]
- Djikanović, D.; Kalauzi, A.; Jeremić, M.; Xu, J.; Mićić, M.; Whyte, J.D.; Leblanc, R.M.; Radotić, K. Interaction of the CdSe quantum dots with plant cell walls. Colloid. Surface. B 2012, 91, 41–47. [Google Scholar] [CrossRef]
- Yang, D.; Moran-Mirabal, J.M.; Parlange, J.Y.; Walker, L.P. Investigation of the porous structure of cellulosic substrates through confocal laser scanning microscopy. Biotechnol. Bioeng. 2013, 110, 2836–2845. [Google Scholar] [CrossRef]
- Paës, G.; Chabbert, B. Characterization of arabinoxylan / cellulose nanocrystals gels to investigate fluorescent probes mobility in bio-inspired models of plant secondary cell wall. Biomacromolecules 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Paës, G.; Burr, S.; Saab, M.-B.; Molinari, M.; Aguié-Béghin, V.; Chabbert, B. Modeling progression of fluorescent probes in bioinspired lignocellulosic assemblies. Biomacromolecules 2013, 14, 2196–2205. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Newman, R.H.; Vaidya, A. Nanoscale interactions of polyethylene glycol with thermo-mechanically pre-treated Pinus radiata biofuel substrate. Biotechnol. Bioeng. 2014, 111, 719–725. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Boratson, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef]
- Arantes, V.; Saddler, J.N. Access to cellulose limits the efficiency of enzymatic hydrolysis: The role of amorphogenesis. Biotech. Biofuels 2010, 3, 1–11. [Google Scholar]
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struct. Biol. 2013, 23, 669–677. [Google Scholar] [CrossRef]
- McCartney, L.; Gilbert, H.J.; Bolam, D.N.; Boraston, A.B.; Knox, J.P. Glycoside hydrolase carbohydrate-binding modules as molecular probes for the analysis of plant cell wall polymers. Anal. Biochem. 2004, 326, 49–54. [Google Scholar]
- Hilden, L.; Daniel, G.; Johansson, G. Use of a fluorescence labelled, carbohydrate-binding module from Phanerochaete chrysosporium Cel7D for studying wood cell wall ultrastructure. Biotechnol. Lett. 2003, 25, 553–558. [Google Scholar] [CrossRef]
- Filonova, L.; Gunnarsson, L.C.; Daniel, G.; Ohlin, M. Synthetic xylan-binding modules for mapping of pulp fibres and wood sections. BMC Plant Biol. 2007, 7, 54. [Google Scholar]
- Ding, S.Y.; Xu, Q.; Ali, M.K.; Baker, J.O.; Bayer, E.A.; Barak, Y.; Lamed, R.; Sugiyama, J.; Rumbles, G.; Himmel, M.E. Versatile derivatives of carbohydrate-binding modules for imaging of complex carbohydrates approaching the molecular level of resolution. Biotechniques 2006, 41, 435–442. [Google Scholar]
- Porter, S.E.; Donohoe, B.S.; Beery, K.E.; Xu, Q.; Ding, S.Y.; Vinzant, T.B.; Abbas, C.A.; Himmel, M.E. Microscopic analysis of corn fiber using starch- and cellulose-specific molecular probes. Biotechnol. Bioeng. 2007, 98, 123–131. [Google Scholar] [CrossRef]
- Kawakubo, T.; Karita, S.; Araki, Y.; Watanabe, S.; Oyadomari, M.; Takada, R.; Tanaka, F.; Abe, K.; Watanabe, T.; Honda, Y. Analysis of exposed cellulose surfaces in pretreated wood biomass using Carbohydrate-Binding Module (CBM)-Cyan Fluorescent Protein (CFP). Biotechnol. Bioeng. 2010, 105, 499–508. [Google Scholar] [CrossRef]
- Dagel, D.J.; Liu, Y.S.; Zhong, L.L.; Luo, Y.H.; Himmel, M.E.; Xu, Q.; Zeng, Y.N.; Ding, S.Y.; Smith, S. In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J. Phys. Chem. B 2011, 115, 635–641. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zeng, Y.N.; Luo, Y.H.; Xu, Q.; Himmel, M.E.; Smith, S.J.; Ding, S.Y. Does the cellulose-binding module move on the cellulose surface? Cellulose 2009, 16, 587–597. [Google Scholar] [CrossRef]
- Jervis, E.J.; Haynes, C.A.; Kilburn, D.G. Surface diffusion of cellulases and their isolated binding domains on cellulose. J. Biol. Chem. 1997, 272, 24016–24023. [Google Scholar] [CrossRef]
- Dornez, E.; Cuyvers, S.; Holopainen, U.; Nordlund, E.; Poutanen, K.; Delcour, J.A.; Courtin, C.M. Inactive fluorescently labeled xylanase as a novel probe for microscopic analysis of arabinoxylan containing cereal cell walls. J. Agric. Food Chem. 2011, 59, 6369–6375. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Walker, L.P.; Moran-Mirabal, J.M. Observing and modeling BMCC degradation by commercial cellulase cocktails with fluorescently labeled Trichoderma reseii Cel7A through confocal microscopy. Biotechnol. Bioeng. 2013, 110, 108–117. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Bolewski, J.C.; Walker, L.P. Thermobifida fusca cellulases exhibit limited surface diffusion on bacterial micro-crystalline cellulose. Biotechnol. Bioeng. 2013, 110, 47–56. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Bolewski, J.C.; Walker, L.P. Reversibility and binding kinetics of Thermobifida fusca cellulases studied through fluorescence recovery after photobleaching microscopy. Biophys. Chem. 2011, 155, 20–28. [Google Scholar] [CrossRef]
- Cuyvers, S.; Hendrix, J.; Dornez, E.; Engelborghs, Y.; Delcour, J.A.; Courtin, C.M. Both substrate hydrolysis and secondary substrate binding determine xylanase mobility as assessed by FRAP. J. Phys. Chem. B 2011, 115, 4810–4817. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, Y.Q.; Ragauskas, A.J. A novel FRET approach for in situ investigation of cellulase-cellulose interaction. Anal. Bioanal. Chem. 2010, 398, 1257–1262. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, Y.Q.; Ragauskas, A.J. Determination of cellulase colocalization on cellulose fiber with quantitative FRET measured by acceptor photobleaching and spectrally unmixing fluorescence microscopy. Analyst 2012, 137, 1319–1324. [Google Scholar] [CrossRef]
- Schermelleh, L.; Heintzmann, R.; Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 2010, 190, 165–175. [Google Scholar] [CrossRef]
- Liesche, J.; Ziomkiewicz, I.; Schulz, A. Super-resolution imaging with Pontamine Fast Scarlet 4BS enables direct visualization of cellulose orientation and cell connection architecture in onion epidermis cells. BMC Plant Biol. 2013, 13, 226. [Google Scholar] [CrossRef]
- Lukyanov, K.A.; Chudakov, D.M.; Lukyanov, S.; Verkhusha, V.V. Photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 2005, 6, 885–891. [Google Scholar] [CrossRef]
- Patterson, G.; Davidson, M.; Manley, S.; Lippincott-Schwartz, J. Superresolution imaging using single-molecule localization. Annu. Rev. Phys. Chem. 2010, 61, 345–367. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paës, G. Fluorescent Probes for Exploring Plant Cell Wall Deconstruction: A Review. Molecules 2014, 19, 9380-9402. https://doi.org/10.3390/molecules19079380
Paës G. Fluorescent Probes for Exploring Plant Cell Wall Deconstruction: A Review. Molecules. 2014; 19(7):9380-9402. https://doi.org/10.3390/molecules19079380
Chicago/Turabian StylePaës, Gabriel. 2014. "Fluorescent Probes for Exploring Plant Cell Wall Deconstruction: A Review" Molecules 19, no. 7: 9380-9402. https://doi.org/10.3390/molecules19079380






