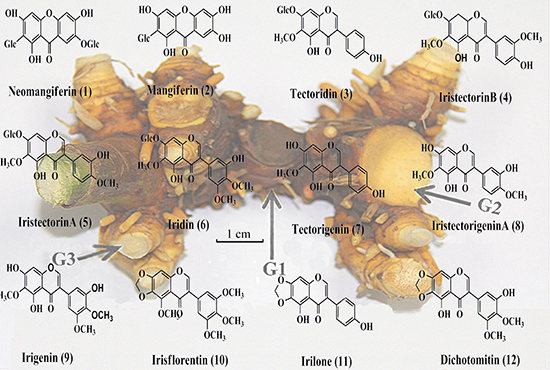
Dynamic Changes of Flavonoids Contents in the Different Parts of Rhizome of Belamcanda chinensis During the Thermal Drying Process
Abstract
:1. Introduction

2. Results and Discussion
2.1. Dehydration Curves
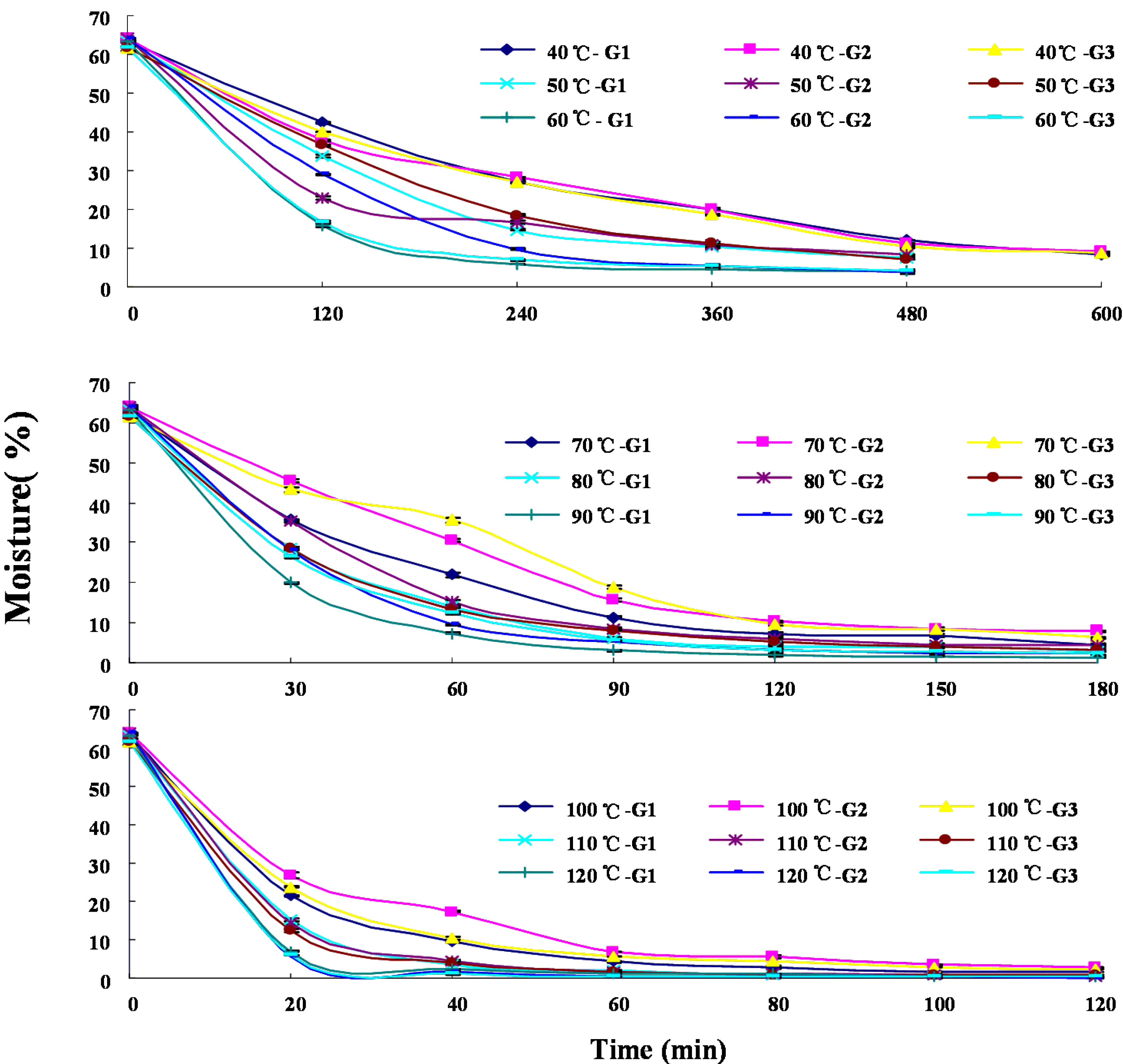
| G. b | T c (°C) | Time (min) | Moist. d | Contents of Analytes (mg/g, DW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| G1 | 40 | 600 | 8.13 ± 0.06 | 1.17 ± 0.10 | 1.23 ± 0.06 | 14.17 ± 0.04 | 1.83 ± 0.02 | 7.74 ± 0.15 | 7.21 ± 0.04 | 1.30 ± 0.09 | 0.60 ± 0.01 | 2.39 ± 0.03 | 0.49 ± 0.01 | 0.14 ± 0.02 | 1.05 ± 0.02 |
| 50 | 420 | 8.41 ± 0.33 | 2.45 ± 0.05 | 1.65 ± 0.01 | 13.32 ± 0.01 | 1.89 ± 0.00 | 6.18 ± 0.19 | 7.19 ± 0.02 | 1.32 ± 0.00 | 0.76 ± 0.01 | 3.13 ± 0.00 | 0.90 ± 0.00 | 0.21 ± 0.01 | 0.92 ± 0.01 | |
| 60 | 180 | 8.53 ± 0.32 | 2.10 ± 0.06 | 1.92 ± 0.08 | 13.72 ± 0.08 | 2.01 ± 0.05 | 6.91 ± 0.07 | 8.35 ± 0.14 | 1.36 ± 0.09 | 0.86 ± 0.05 | 3.71 ± 0.23 | 1.10 ± 0.08 | 0.27 ± 0.01 | 1.00 ± 0.00 | |
| 70 | 120 | 7.35 ± 0.40 | 2.10 ± 0.23 | 1.65 ± 0.07 | 11.79 ± 0.13 | 1.73 ± 0.02 | 5.83 ± 0.03 | 7.04 ± 0.00 | 1.19 ± 0.09 | 0.70 ± 0.00 | 3.13 ± 0.08 | 0.93 ± 0.00 | 0.19 ± 0.02 | 0.81 ± 0.01 | |
| 80 | 90 | 6.06 ± 0.31 | 1.79 ± 0.03 | 2.09 ± 0.14 | 13.47 ± 0.03 | 2.06 ± 0.08 | 6.36 ± 0.04 | 7.72 ± 0.10 | 1.23 ± 0.05 | 0.87 ± 0.05 | 3.73 ± 0.22 | 1.28 ± 0.10 | 0.30 ± 0.00 | 1.16 ± 0.06 | |
| 90 | 60 | 7.33 ± 0.19 | 1.73 ± 0.12 | 1.59 ± 0.08 | 13.69 ± 0.26 | 2.01 ± 0.07 | 6.12 ± 0.07 | 7.48 ± 0.09 | 1.04 ± 0.03 | 0.79 ± 0.04 | 3.36 ± 0.06 | 1.06 ± 0.05 | 0.26 ± 0.00 | 0.91 ± 0.03 | |
| 100 | 40 | 9.69 ± 0.35 | 1.69 ± 0.03 | 1.74 ± 0.00 | 13.25 ± 0.03 | 2.07 ± 0.17 | 7.13 ± 0.13 | 8.30 ± 0.01 | 1.40 ± 0.06 | 0.97 ± 0.00 | 4.17 ± 0.01 | 1.38 ± 0.01 | 0.33 ± 0.01 | 1.25 ± 0.02 | |
| 110 | 40 | 3.57 ± 0.16 | 1.83 ± 0.01 | 1.64 ± 0.01 | 12.34 ± 0.00 | 1.90 ± 0.01 | 6.05 ± 0.04 | 7.94 ± 0.01 | 1.03 ± 0.04 | 0.79 ± 0.02 | 3.39 ± 0.06 | 1.00 ± 0.02 | 0.24 ± 0.00 | 0.91 ± 0.00 | |
| 120 | 20 | 7.00 ± 0.35 | 1.61 ± 0.07 | 1.85 ± 0.03 | 12.20 ± 0.04 | 1.90 ± 0.09 | 6.09 ± 0.03 | 7.04 ± 0.05 | 1.03 ± 0.03 | 0.87 ± 0.07 | 3.42 ± 0.05 | 0.91 ± 0.17 | 0.25 ± 0.04 | 0.71 ± 0.04 | |
| G2 | 40 | 600 | 9.03 ± 0.12 | 0.99 ± 0.00 | 1.11 ± 0.07 | 15.66 ± 0.27 | 1.81 ± 0.01 | 6.59 ± 0.11 | 5.97 ± 0.01 | 1.03 ± 0.02 | 0.61 ± 0.03 | 1.86 ± 0.00 | 0.40 ± 0.01 | 0.13 ± 0.01 | 0.67 ± 0.00 |
| 50 | 420 | 8.40 ± 0.15 | 0.98 ± 0.01 | 1.46 ± 0.01 | 12.11 ± 0.18 | 1.66 ± 0.03 | 6.20 ± 0.06 | 6.65 ± 0.03 | 0.88 ± 0.03 | 0.63 ± 0.01 | 2.34 ± 0.06 | 0.67 ± 0.02 | 0.18 ± 0.01 | 0.55 ± 0.02 | |
| 60 | 240 | 9.62 ± 0.17 | 1.12 ± 0.00 | 1.25 ± 0.05 | 14.59 ± 0.29 | 1.84 ± 0.01 | 6.99 ± 0.12 | 7.32 ± 0.14 | 1.15 ± 0.01 | 0.70 ± 0.02 | 2.47 ± 0.06 | 0.64 ± 0.01 | 0.16 ± 0.01 | 0.73 ± 0.01 | |
| 70 | 150 | 8.39 ± 0.23 | 1.01 ± 0.01 | 1.28 ± 0.01 | 11.86 ± 0.27 | 1.59 ± 0.01 | 6.40 ± 0.13 | 6.69 ± 0.10 | 1.04 ± 0.02 | 0.73 ± 0.02 | 2.61 ± 0.06 | 0.64 ± 0.01 | 0.16 ± 0.00 | 0.62 ± 0.01 | |
| 80 | 90 | 8.51 ± 0.31 | 1.13 ± 0.04 | 1.62 ± 0.03 | 11.48 ± 0.32 | 1.73 ± 0.05 | 6.28 ± 0.17 | 7.07 ± 0.14 | 1.15 ± 0.02 | 0.83 ± 0.03 | 3.18 ± 0.02 | 0.88 ± 0.01 | 0.20 ± 0.01 | 0.96 ± 0.04 | |
| 90 | 60 | 9.53 ± 0.23 | 1.10 ± 0.05 | 1.22 ± 0.03 | 11.56 ± 0.12 | 1.62 ± 0.01 | 5.66 ± 0.03 | 6.25 ± 0.00 | 1.22 ± 0.01 | 0.83 ± 0.02 | 2.78 ± 0.04 | 0.73 ± 0.02 | 0.18 ± 0.01 | 0.73 ± 0.01 | |
| 100 | 60 | 6.78 ± 0.11 | 1.02 ± 0.02 | 1.56 ± 0.04 | 11.55 ± 0.14 | 1.48 ± 0.03 | 6.14 ± 0.16 | 6.44 ± 0.11 | 0.98 ± 0.01 | 0.85 ± 0.03 | 3.40 ± 0.06 | 1.02 ± 0.04 | 0.25 ± 0.01 | 0.80 ± 0.01 | |
| 110 | 40 | 4.41 ± 0.38 | 1.23 ± 0.01 | 1.54 ± 0.02 | 11.71 ± 0.34 | 1.62 ± 0.03 | 5.86 ± 0.10 | 6.17 ± 0.16 | 0.92 ± 0.01 | 0.68 ± 0.02 | 2.73 ± 0.02 | 0.76 ± 0.02 | 0.20 ± 0.01 | 0.74 ± 0.03 | |
| 120 | 20 | 5.53 ± 0.31 | 1.18 ± 0.03 | 1.03 ± 0.07 | 11.34 ± 0.29 | 1.55 ± 0.04 | 5.52 ± 0.13 | 6.89 ± 0.11 | 1.06 ± 0.03 | 0.78 ± 0.02 | 1.86 ± 0.04 | 0.77 ± 0.03 | 0.19 ± 0.01 | 0.56 ± 0.02 | |
| G3 | 40 | 600 | 8.57 ± 0.13 | 1.08 ± 0.01 | 1.53 ± 0.00 | 13.64 ± 0.01 | 1.75 ± 0.00 | 6.28 ± 0.03 | 5.81 ± 0.02 | 1.38 ± 0.11 | 0.69 ± 0.00 | 2.85 ± 0.07 | 0.50 ± 0.02 | 0.14 ± 0.00 | 0.49 ± 0.04 |
| 50 | 420 | 7.57 ± 0.09 | 1.1 ± 0.05 | 1.9 ± 0.00 | 11.5 ± 0.00 | 1.7 ± 0.00 | 6.4 ± 0.00 | 6.4 ± 0.00 | 1.2 ± 0.00 | 0.8 ± 0.00 | 3.2 ± 0.01 | 0.6 ± 0.01 | 0.2 ± 0.00 | 0.6 ± 0.00 | |
| 60 | 240 | 7.01 ± 0.16 | 1.06 ± 0.00 | 1.96 ± 0.00 | 11.20 ± 0.00 | 1.70 ± 0.00 | 6.08 ± 0.00 | 6.04 ± 0.00 | 1.43 ± 0.00 | 0.83 ± 0.00 | 3.73 ± 0.00 | 0.71 ± 0.00 | 0.18 ± 0.00 | 0.74 ± 0.00 | |
| 70 | 120 | 9.51 ± 0.43 | 1.38 ± 0.01 | 1.68 ± 0.18 | 10.85 ± 0.10 | 1.44 ± 0.14 | 6.25 ± 0.06 | 5.58 ± 0.05 | 1.55 ± 0.19 | 0.88 ± 0.09 | 3.86 ± 0.05 | 0.59 ± 0.05 | 0.20 ± 0.00 | 0.71 ± 0.00 | |
| 80 | 90 | 8.16 ± 0.31 | 1.00 ± 0.05 | 1.61 ± 0.06 | 11.41 ± 0.50 | 1.58 ± 0.04 | 6.02 ± 0.23 | 5.65 ± 0.05 | 1.14 ± 0.06 | 0.78 ± 0.03 | 3.26 ± 0.01 | 0.55 ± 0.01 | 0.17 ± 0.00 | 0.62 ± 0.00 | |
| 90 | 90 | 5.44 ± 0.30 | 1.05 ± 0.01 | 1.82 ± 0.11 | 11.24 ± 0.03 | 1.79 ± 0.06 | 7.18 ± 0.02 | 6.86 ± 0.14 | 1.07 ± 0.08 | 0.81 ± 0.03 | 3.63 ± 0.29 | 0.69 ± 0.08 | 0.20 ± 0.03 | 0.59 ± 0.04 | |
| 100 | 60 | 5.62 ± 0.15 | 0.98 ± 0.03 | 1.56 ± 0.12 | 10.64 ± 0.08 | 1.57 ± 0.05 | 6.50 ± 0.04 | 5.96 ± 0.14 | 1.32 ± 0.03 | 0.90 ± 0.05 | 3.94 ± 0.25 | 0.72 ± 0.02 | 0.21 ± 0.00 | 0.87 ± 0.00 | |
| 110 | 40 | 4.00 ± 0.25 | 0.96 ± 0.03 | 1.82 ± 0.08 | 10.52 ± 0.19 | 1.59 ± 0.04 | 6.80 ± 0.15 | 6.89 ± 0.23 | 1.11 ± 0.07 | 0.87 ± 0.08 | 3.71 ± 0.03 | 0.79 ± 0.10 | 0.21 ± 0.01 | 0.93 ± 0.01 | |
| 120 | 20 | 5.99 ± 0.19 | 0.99 ± 0.04 | 1.49 ± 0.00 | 10.30 ± 0.44 | 1.52 ± 0.09 | 6.38 ± 0.19 | 6.15 ± 0.20 | 0.89 ± 0.06 | 0.71 ± 0.03 | 3.31 ± 0.11 | 0.67 ± 0.04 | 0.20 ± 0.00 | 0.68 ± 0.01 | |
2.2. Determination of Analytes
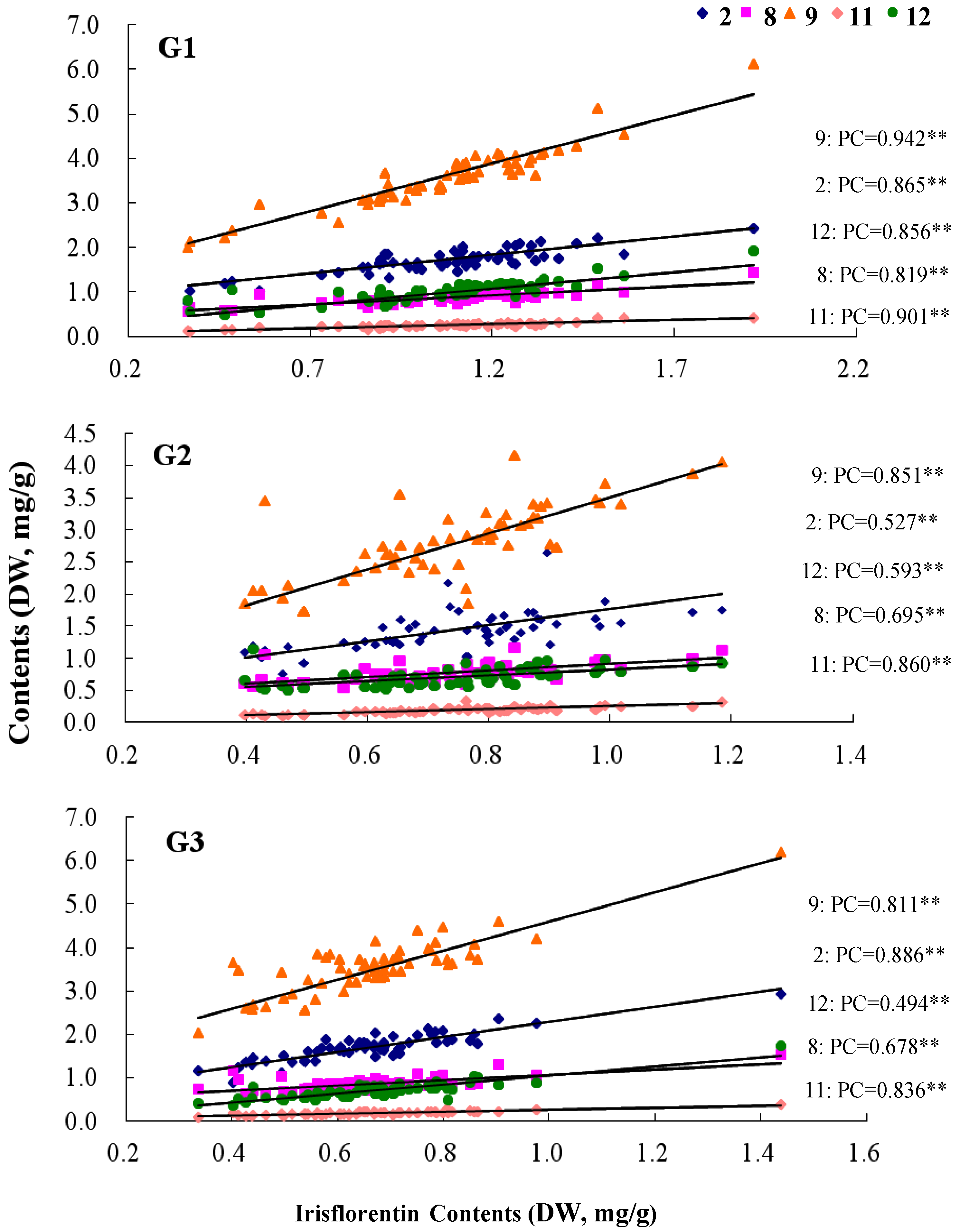
2.3. Differences of the Flavonoids Contents in Three Subunit Parts of Belamcandae Rhizoma
2.4. Dynamic Changes of Flavonoids in Thermal Drying Process
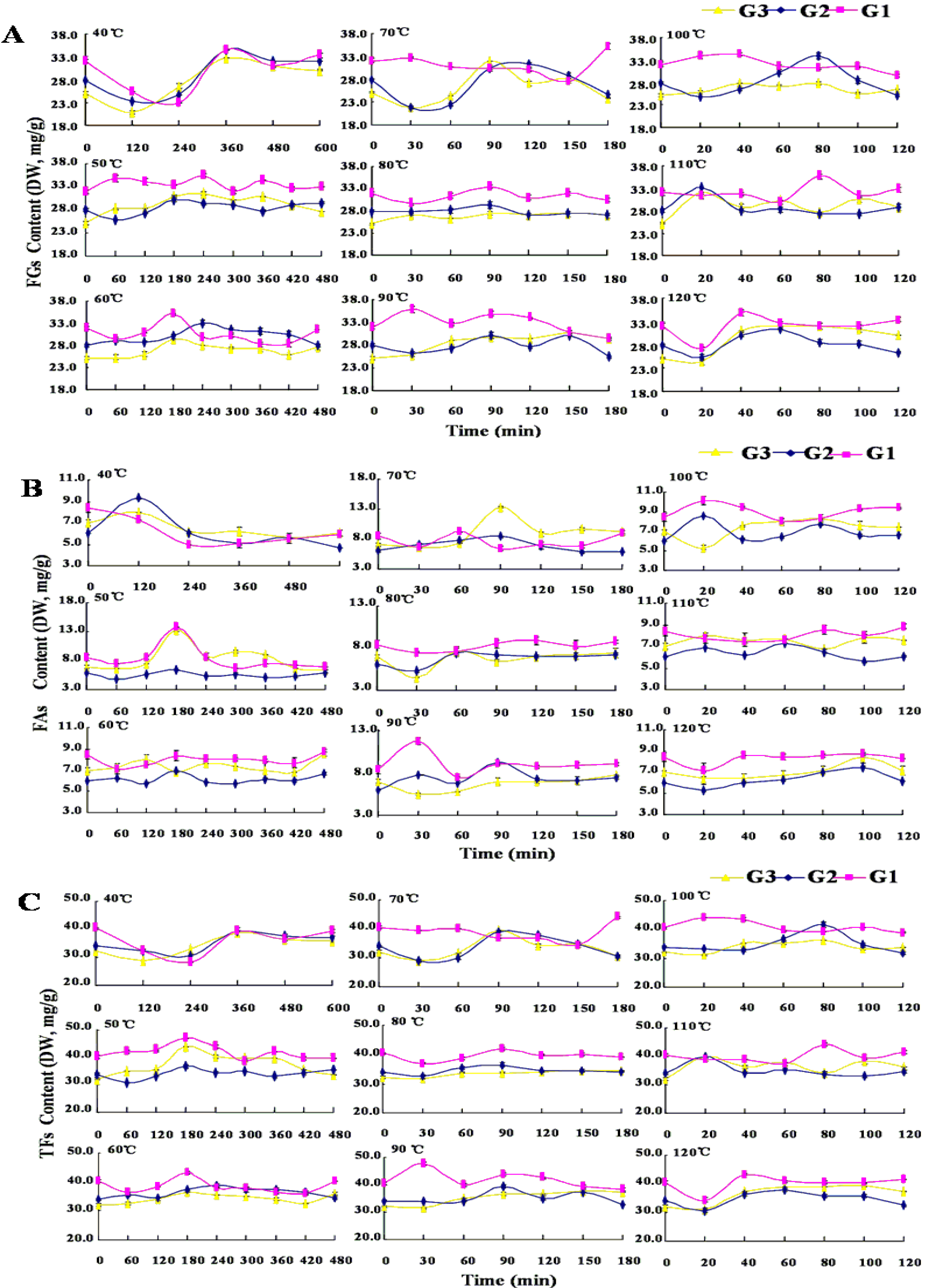
| CF a | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | 100 °C | 110 °C | 120 °C | |
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | FGs (%) | 4.28 | 2.16 | 9.47 | −5.75 | 4.68 | 1.98 | 6.88 | −0.85 | −14.85 |
| FAs (%) | −29.39 | −14.24 | −1.59 | −17.68 | 1.46 | −12.14 | 12.53 | −12.84 | −14.79 | |
| TFs (%) | −2.75 | −1.26 | 7.16 | −8.24 | 4.01 | −0.97 | 8.06 | −3.36 | −14.84 | |
| G2 | FGs (%) | 14.75 | 3.81 | 18.28 | 2.93 | 4.69 | −2.07 | 8.42 | 0.47 | −9.63 |
| FAs (%) | −21.90 | −12.39 | −2.60 | −3.41 | 19.83 | 12.26 | 6.66 | 2.93 | −13.17 | |
| TFs (%) | 8.28 | 0.94 | 14.59 | 1.81 | 7.36 | 0.46 | 8.11 | 0.91 | −10.25 | |
| G3 | FGs (%) | 19.79 | 15.08 | 11.67 | 8.21 | 8.59 | 19.21 | 8.30 | 13.78 | −3.11 |
| FAs (%) | −13.56 | −5.48 | 8.88 | 27.34 | −6.80 | 0.02 | 13.90 | 8.90 | −7.65 | |
| TFs (%) | 12.54 | 10.61 | 11.06 | 6.70 | 5.24 | 15.03 | 9.52 | 12.71 | −4.10 |
3. Experimental Section
3.1. Plant Materials
3.2. Drying Process
3.3. Determination of Moisture
3.4. Chemicals and Reagents
3.5. Sample Preparation
3.6. Chromatographic Conditions
3.7. Calibration and Method Validation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2010; Volume 1, pp. 267–268. [Google Scholar]
- Ha, S.C.; Won, S.W. Flavonoids from the rhizomes of Belamcanda chinesis. Arch. Pharm. Res. 1991, 14, 357–358. [Google Scholar] [CrossRef]
- Woo, W.S.; Woo, E.H. An Isoflavone noririsfiorentin from Belamcanda chinensis. Phytochemistry 1993, 33, 939–940. [Google Scholar] [CrossRef]
- Ito, H.; Onoue, S.; Yoshida, T. Isoflavonoids from Belamcanda chinensis. Chem. Pharm. Bull. 2001, 49, 1229–1231. [Google Scholar] [CrossRef]
- Ji, W.L.; Qin, M.J.; Wang, Z.T. Studies on the constituents of Belamcanda chinensis (Ι). J. China Pharm. Univ. 2001, 32, 197–199. [Google Scholar]
- Qin, M.J.; Ji, W.L.; Wang, Z.T. Study on chemical constituents of Belamcanda chinensis (Ι). Chin. Tradit. Herb. Drugs 2004, 35, 487–489. [Google Scholar]
- Seidlova, W.D.; Hesse, O.; Jarry, H.; Rimoldi, G.; Thelen, P.; Christoffel, V.; Wuttke, W. Belamcanda chinensis and the thereof purified tectorigenin have selective estrogen receptor modulator activities. Phytomedicine 2004, 11, 392–403. [Google Scholar]
- Li, J.; Li, W.Z.; Huang, W.; Cheung, A.W.; Bi, C.W.; Duan, R.; Guo, A.J.; Dong, T.T.; Tsim, K.W. Quality evaluation of Rhizoma Belamcanda (Belamcanda chinensis (L.) DC.) by using high-performance liquid chromatography coupled with diode array detector and mass spectrometry. J. Chromatogr. A 2009, 1216, 2071–2078. [Google Scholar] [CrossRef]
- Qin, M.J.; Tanaka, T.; Yu, G.D.; Ji, W.L.; Wang, Z.T. Biological Research of Belamcanda chinensis. J. Chin. Med. Mater. 2003, 1, 4–5. [Google Scholar]
- Li, P.; Qi, L.W.; Wen, X.D.; Sheng, L.H. Methods for the elucidation of bioactive components and quality control of Traditional Chinese Medicines. Chin. J. Nat. Med. 2007, 5, 1–9. [Google Scholar]
- Pan, L.H.; Li, X.F.; Wang, M.N.; Zha, X.Q.; Yang, X.F.; Liu, Z.J.; Luo, Y.B.; Luo, J.P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef]
- Wan, J.B.; Yang, F.Q.; Li, S.P.; Wang, Y.T.; Cui, X.M. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J. Pharm. Biomed. 2006, 41, 1596–1601. [Google Scholar] [CrossRef]
- Peng, R.; Ma, P.; Mo, R.; Sun, N. Analysis of the bioactive components from different growth stages of Fritillaria taipaiensis P.Y. Li. Acta Pharm. Sin. B 2013, 3, 167–173. [Google Scholar] [CrossRef]
- Claudia, D.B.; Silvia, T.; Marco, M.; Luciana, G.A. Changes in soil chemical parameters and organic matter balance after 13 years of ramie [Boehmeria nivea (L.) Gaud.] cultivation in the Mediterranean region. Eur. J. Agron. 2011, 35, 154–163. [Google Scholar]
- Mhamdi, B.; Aidi Wannes, W.; Sriti, J.; Jellali, I.; Ksouri, R.; Marzouk, B. Effect of harvesting time on phenolic compounds and antiradical scavenging activity of Borago officinalis seed extracts. Ind. Crop. Prod. 2010, 31, e1–e4. [Google Scholar] [CrossRef]
- Shan, G.S.; Zhang, L.X.; Zhao, Q.M.; Xiao, H.B.; Zhuo, R.J.; Xu, G.; Jiang, H.; You, X.M.; Jia, T.Z. Metabolomic study of raw and processed Atractylodes Macrocephala Koidz by LC-MS. J. Pharm. Biomed. Anal. 2014, 16, 74–84. [Google Scholar]
- Rinaldi, R.; Amodio, M.L.; Colelli, G. Effect of temperature and exogenous ethylene on the physiological and quality traits of purslane (Portulaca oleracea L.) leaves during storage. Postharvest Biol. Technol. 2010, 58, 147–156. [Google Scholar] [CrossRef]
- Davidson, V.; Li, X.; Brown, R. Forced-air drying of ginseng root: 1. Effects of air temperature on quality. J. Food Eng. 2004, 63, 361–367. [Google Scholar]
- Tankoa, H.; Carriera, D.J.; Duana, L.; Clausena, E. Pre-and post harvest processing of medicinal plants. Plant Genet. Res. 2005, 3, 304–313. [Google Scholar] [CrossRef]
- Martynenko, A.I.; Brown, R.B.; Davidson, V.J. Physical and physiological factors of ginseng drying. Appl. Eng. Agric. 2006, 22, 571–576. [Google Scholar]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; Oriordan, D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT Food Sci. Technol. 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Li, X.B.; Wang, W.; Zhou, G.J.; Li, Y.; Xie, X.M.; Zhou, T.S. Production of salvianolic acid in rootsof Salvia miltiorrhiza (Danshen) during the harvest drying process. Molecules 2012, 17, 2388–2407. [Google Scholar] [CrossRef]
- Morrissey, C.; Bektic, J.; Spengler, B.; Galvin, D.; Christoffel, V.; Klocker, H.; Fitzpatrick, J.M. Phytoestrogens derived from Belamcanda chinensis have an antiproliferative effect on prostate cancer cells in vitro. J. Urol. 2004, 172, 2426–2433. [Google Scholar] [CrossRef]
- Wozniak, D.; Janda, B.; Kapusta, I. Antimutagenic and anti-oxidant activities of isoflavonoids from of Belamcanda chinensis (L.) DC. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2010, 696, 148–153. [Google Scholar]
- Hasibeder, A.; Venkataramani, V.; Thelen, P. Phytoestrogens regulate the proliferation and expression of stem cell factors in cell lines of malignant testicular germ cell tumors. Int. J. Oncol. 2013, 43, 1385–1394. [Google Scholar]
- Wang, Y.J.; Peng, H.S.; Shen, Y.; Zhao, R.; Huang, L.Q. The profiling of bioactive ingredients of differently aged Salvia miltiorrhiza roots. Microsc. Res. Tech. 2013, 76, 947–954. [Google Scholar] [CrossRef]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; van Montagu, M.; Inzé, D.; van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000, 57, 779–795. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Qin, M.J.; Ji, W.L.; Liu, J.; Zhao, J.; Yu, G.D. Scavenging effects on radicals of isoflavones from rhizome of Belamcanda chinensis. Chin. Tradit. Herb. Drugs 2003, 34, 640–642. [Google Scholar]
- Shu, P. Quality Evaluation of Several Medicinal Plants from Iridaceae and the Pharmacological Efficacy Studies on Main Bioactive Constituents. Ph.D. Thesis, 2010; pp. 132–148. [Google Scholar]
- Chang, C.H.; Lin, H.Y.; Chang, C.Y.; Chuan, L.Y. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Ren, G.; Chen, F. Drying of North American Ginseng (Panax quinquefolium) roots by microwave-hot air combination. J. Food Eng. 1998, 35, 433–443. [Google Scholar]
- Yabar, E.; Pedreschi, R.; Chirinose, R.; Campos, D. Glucosinolate content and myrosinase activity evolution in three maca (Lepidium meyenii Walp.) ecotypes during preharvest, harvest and postharvest drying. Food Chem. 2011, 127, 1576–1583. [Google Scholar] [CrossRef]
- Shang, H.Q.; Qin, M.J.; Wu, J.R. Constituents of rhizomes of Iris tectorum. Chin. J. Nat. Med. 2007, 5, 312–314. [Google Scholar]
- Sample Availability: Samples of tectoridin, iridin, tectorigenin, irigenin are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Pu, B.-Q.; Xie, G.-Y.; Tian, M.; Xu, F.-Y.; Qin, M.-J. Dynamic Changes of Flavonoids Contents in the Different Parts of Rhizome of Belamcanda chinensis During the Thermal Drying Process. Molecules 2014, 19, 10440-10454. https://doi.org/10.3390/molecules190710440
Zhu Y, Pu B-Q, Xie G-Y, Tian M, Xu F-Y, Qin M-J. Dynamic Changes of Flavonoids Contents in the Different Parts of Rhizome of Belamcanda chinensis During the Thermal Drying Process. Molecules. 2014; 19(7):10440-10454. https://doi.org/10.3390/molecules190710440
Chicago/Turabian StyleZhu, Yan, Bing-Qing Pu, Guo-Yong Xie, Mei Tian, Fang-Yun Xu, and Min-Jian Qin. 2014. "Dynamic Changes of Flavonoids Contents in the Different Parts of Rhizome of Belamcanda chinensis During the Thermal Drying Process" Molecules 19, no. 7: 10440-10454. https://doi.org/10.3390/molecules190710440