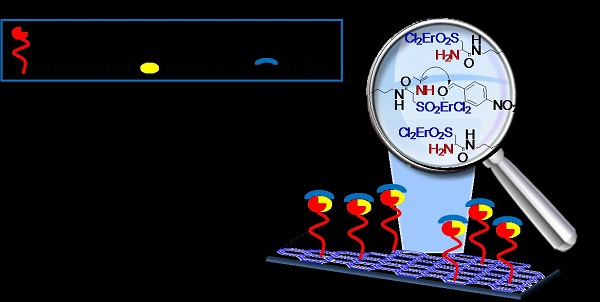
An Erbium-Based Bifuctional Heterogeneous Catalyst: A Cooperative Route Towards C-C Bond Formation
Abstract
:1. Introduction
2. Results and Discussion


| Total [-NH2] (mmol/gr) | AA [-NH2] (mmol/gr) | [Erm] (mmol/gr) | |
|---|---|---|---|
| Cys 01 | 1.00 | 1.00 | - |
| Cys 02 | 1.00 | 1.00 | - |
| Er-Cys05 | 0.94 | 0.94 | 0.94 |
| Entry | Compound | Total [-NH2] (mmol/gr) | [ErIII] (mmol/gr) | Textural Properties | FT–IR  (cm−1) (cm−1) | ||
|---|---|---|---|---|---|---|---|
| SBET (m2/gr) | BJH Pore Volume (cm3/gr) | BJH Average Pore Diameter (Å) | |||||
| 1 | MCM-41 | - | - | 1600 | 1.70 | 35 | 452 (s, νs (Si-OSi)), 3458 (vs, νs (HO-H)) |
| 2 | Cys 01 | 1.00 | - | Ref30 a | Ref30 a | Ref30 a | 461 (s, νs (Si-OSi)), 1630 (b, N-H), 2938 (vs C-H), 3446 (vs, νs (HO-H)) |
| 3 | Cys 02 | 1.00 | - | - | - | - | - |
| 4 | Cys 03 | 0.94 | - | - | - | - | - |
| 5 | Er-Cys 04 | 0.94 | 0.94 | - | - | - | - |
| 6 | Er-Cys05 | 0.94 | 0.94 | 327 | 0.24 | 40 | 465 (s, νs (Si-OSi)), 791 (m, νs (C-S)), 1312 (w, νs (S=O)), 1550 (w, δ (N-H/C-N)), 1650 (s, νs (C=O)), 2342 (w, νs (N-H3+)), 3442 (vs, νs (HO–H)) |
Characteristics of Catalyst Surface were Monitored by N2 Adsorption-Desorption Technique
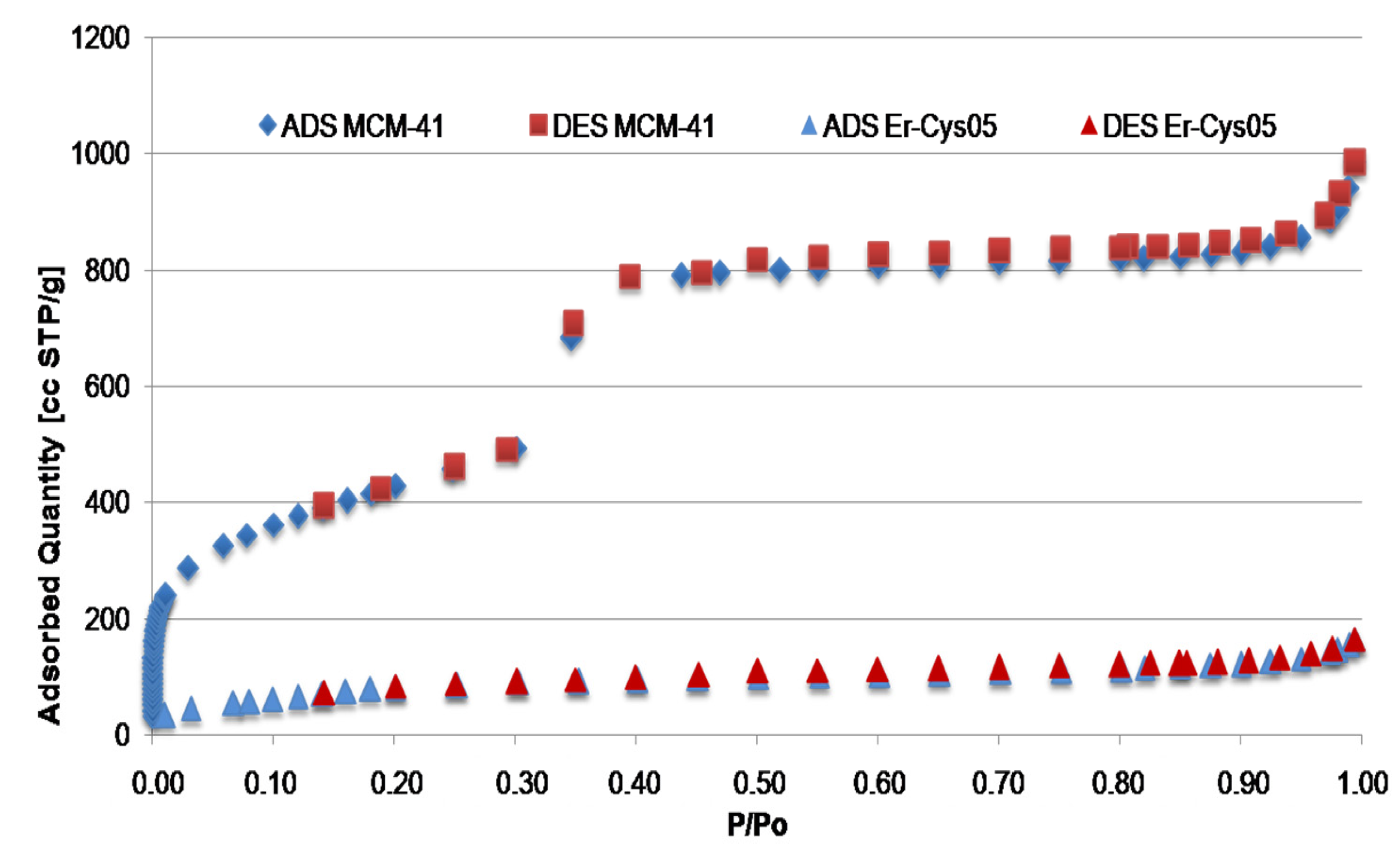
| Catalyst | S BET (m2/gr) | Pore Volume (cm3/gr) | Average Pore Diameter (Å) |
|---|---|---|---|
| MCM-41 | 1600 | 1.70 | 35 |
| Er-Cys05 | 330 | 0.24 | 40 |

| Entry | Catalyst a | Henry Reaction (1) | Aldol Reaction (2a) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yield (%) | TON b | TOF b | Yield (%) | TON b | TOF b | ||||
| 1 | Er-Cys05 | 62 | 3.5 | 0.17 | 90 | 6.7 | 0.39 | ||
| 59 c | 4.41 | 0.22 | 87 c | 6.3 | 0.38 | ||||
| 57 d | 3.7 | 0.18 | 82 d | 4.8 | 0.28 | ||||
| 2 | MCM-Er | 16 | 1.0 | 0.05 | – | – | – | ||
| 3 | MCM-NH2 | 10 e | 0.6 | 0.03 | 43 | 3.1 | 0.18 | ||
| 4 | MCM-Er/MCM-NH2 | 25 f | 1.5 | 0.07 | 30 | 2.1 | 0.12 | ||
| 5 | Propylamine/ErCl3 | 10 | 0.6 | 0.03 | 25 | 1.8 | 0.10 | ||
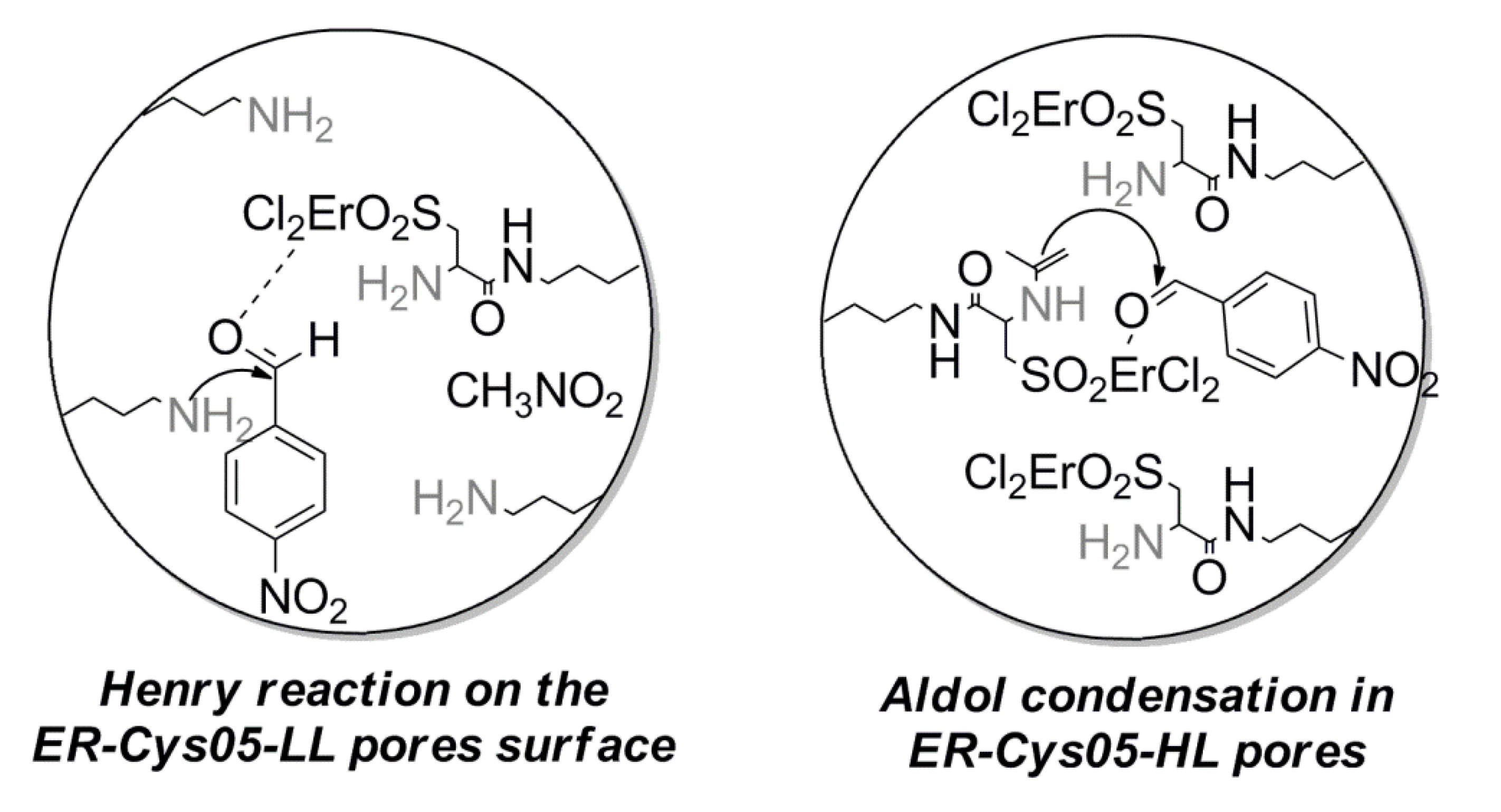
3. Experimental
3.1. General Information
3.2. Synthesis of Er-Cys05
3.3. Aldol Reaction
3.4. Henry Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- France, S.; Shah, M.H.; Weatherwax, A.; Wack, H.; Roth, J.P.; Lectka, T. Bifunctional lewis acid-nucleophile-based asymmetric catalysis: Mechanistic evidence for imine activation working in tandem with chiral enolate formation in the synthesis of β-lactams. J. Am. Chem. Soc. 2005, 127, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Sasai, H.; Arai, T.; Satow, Y.; Hounk, K.N.; Shibasaki, M. The first heterobimetallic multifunctional asymmetric catalyst. J. Am. Chem. Soc. 1995, 117, 6194–6198. [Google Scholar] [CrossRef]
- Aggarwal, V. Metal- and ligand-accelerated catalysis of the baylis-hillman reaction. J. Org. Chem. 1998, 63, 7183–7189. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Chen, H.-T.; Wiench, J.W.; Pruski, M.; Lin, V.S.-Y. Cooperative catalysis by general acid and base bifunctionalized mesoporous silica nanospheres. Angew. Chem. Int. Ed. 2005, 44, 1826–1830. [Google Scholar] [CrossRef]
- Zeidan, R.K.; Hwang, S.-J.; Davis, M.E. Multifunctional heterogeneous catalysts: SBA-15-containing primary amines and sulfonic acids. Angew. Chem. Int. Ed. 2006, 45, 6332–6335. [Google Scholar] [CrossRef]
- Bass, J.D.; Solovyov, A.; Pascall, A.J.; Katz, A. Acid-base bifunctional and dielectric outer-sphere effects in heterogeneous catalysis: A comparative investigation of model primary amine catalysts. J. Am. Chem. Soc. 2006, 128, 3737–3747. [Google Scholar] [PubMed]
- Motokura, K.; Tada, M.; Iwasawa, Y. Cooperative catalysis of primary and tertiary amines immobilized on oxide surfaces for one-pot C[BOND]C bond forming reactions. Angew. Chem. Int. Ed. 2008, 47, 9230–9235. [Google Scholar] [CrossRef]
- Motokura, K.; Tanaka, S.; Tada, M.; Iwasawa, Y. Bifunctional heterogeneous catalysis of silica-alumina-supported tertiary amines with controlled acid-base interactions for efficient 1,4-addition reactions. Chem. Eur. J. 2009, 15, 10871–10879. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guan, J.; Wu, S.; Liu, H.; Liu, B.; Kan, Q. Synthesis,characterization and catalytic activity of acid-base bifunctional materials by controlling steric hindrance. Micropor. Mesopor. Mat. 2010, 128, 120–125. [Google Scholar] [CrossRef]
- Jaroniek, M. Materials science: Organosilica the conciliator. Nature 2006, 442, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, X.; Wu, S.; Liu, B.; Liu, H.; Guan, J.; Kan, Q. The effect of the distance between acidic site and basic site immobilized on mesoporous solid on the activity in catalyzing aldol condensation. J. Solid State Chem. 2011, 184, 289–295. [Google Scholar] [CrossRef]
- Shiju, N.R.; Alberts, A.H.; Khalid, S.; Brown, D.R.; Rothenberg, G. Mesoporous silica with site-isolated amine and phosphotungstic acid groups: A solid catalyst with tunable antagonistic functions for one-pot tandem reactions. Angew. Chem. Int. Ed. 2011, 50, 9615–9619. [Google Scholar] [CrossRef]
- Motokura, K.; Tada, M.; Iwasawa, Y. Organofunctionalized catalyst surfaces highly active and selective for carbon-carbon bond-forming reactions. Catal. Today 2009, 147, 203–210. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Pathak, K.; Jasra, R.V.; Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R. Chiral lanthanum-lithium-binaphthol complex covalently bonded to silica and MCM-41 for enantioselective nitroaldol (Henry) reaction. J. Mol. Catal. A Chem. 2006, 244, 110–117. [Google Scholar] [CrossRef]
- Bellemare, A. Continuous-wave silica-based erbium-doped fibre lasers. Prog. Quantum Electron. 2003, 27, 211–266. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Nardi, M.; Oliverio, M.; Paonessa, R.; Procopio, A. Erbium(III) triflate is a highly efficient catalyst for the synthesis of β-alkoxy alcohols,1,2-diols and β-hydroxy sulfides by ring opening of epoxides. Synthesis 2009, 20, 3433–3438. [Google Scholar]
- Procopio, A.; Costanzo, P.; Dalpozzo, R.; Maiuolo, L.; Nardi, M.; Oliverio, M. Efficient ring opening of epoxides with trimethylsilyl azide and cyanide catalyzed by erbium (iii) triflate. Tetrahedron Lett. 2010, 51, 5150–5153. [Google Scholar] [CrossRef]
- Procopio, A.; Russo, B.; Oliverio, M.; Dalpozzo, R.; de Nino, A.; Maiuolo, L.; Tocci, A. Er(III) chloride: A very active acylation catalyst. Aust. J. Chem. 2007, 60, 75–79. [Google Scholar]
- Shannon, R.D. Crystal physics,diffraction,theoretical and general crystallography. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tiseni, P.S.; Peters, R. Catalytic asymmetric formation of δ-lactones from unsaturated acyl halides. Chem. Eur. J. 2010, 16, 2503–2517. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; de Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar]
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A mesoporous ErIII-MCM-41 catalyst for the cyanosilylation of aldehydes and ketones under solvent-free conditions. ChemSusChem. 2008, 1, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Procopio, A.; Glasnov, T.N.; Goessler, W.; Kappe, C.O. Microwave-assisted grafting to MCM-41 silica and its application as catalyst in flow chemistry. Aust. J. Chem. 2011, 64, 1522–1529. [Google Scholar] [CrossRef]
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An eco-sustainable erbium(iii)-catalysed method for formation/cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. [Google Scholar] [CrossRef]
- Goodman, M.; Toniolo, C.; Moroder, L.; Felix, A. Houben-Weyl: Synthesis of Peptides and Peptidomimetics; Thieme Stuttgart: New York, NY, USA, 2002; Volume 1. [Google Scholar]
- Bourel, L.; Carion, O.; Grass-Masse, H.; Melnyk, O. The deprotection of Lys(Mtt) revisited. J. Pept. Sci. 2000, 6, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, L.; Procopio, A.; Oliverio, M.; Paonessa, R.; Prete, R.; Nardi, M.; Casula, M.F.; Testa, F.; Nagy, J.B. Hybrid MCM-41 grafted by a general microwave-assisted procedure: A characterization study. J. Porous Mater. 2013, 20, 865–873. [Google Scholar]
- Shylesh, S.; Wagner, A.; Seifert, A.; Ernst, S.; Thiel, W.R. Cooperative acid-base effects with functionalized mesoporous silica nanoparticles: Applications in carbon-carbon bond-formation reactions. Chem. Eur. J. 2009, 15, 7052–7062. [Google Scholar] [CrossRef] [PubMed]
- Kandel, K.; Althaus, S.M.; Peeraphatdit, C.; Kobayashi, T.; Trewyn, B.G.; Pruski, M.; Slowing, I.I. Substrate inhibition in the heterogeneous catalyzed aldol condensation: A mechanistic study of supported organocatalysts. J. Catal. 2012, 291, 63–68. [Google Scholar] [CrossRef]
- Shang, F.; Liu, H.; Sun, J.; Liu, B.; Wang, C.; Guan, J.; Kan, Q. Synthesis,characterization and catalytic application of bifunctional catalyst: Al-MCM-41-NH2. Catal. Comm. 2011, 12, 739–743. [Google Scholar] [CrossRef]
- Sample Availability: Sample of Er-Cys05 is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. An Erbium-Based Bifuctional Heterogeneous Catalyst: A Cooperative Route Towards C-C Bond Formation. Molecules 2014, 19, 10218-10229. https://doi.org/10.3390/molecules190710218
Oliverio M, Costanzo P, Macario A, De Luca G, Nardi M, Procopio A. An Erbium-Based Bifuctional Heterogeneous Catalyst: A Cooperative Route Towards C-C Bond Formation. Molecules. 2014; 19(7):10218-10229. https://doi.org/10.3390/molecules190710218
Chicago/Turabian StyleOliverio, Manuela, Paola Costanzo, Anastasia Macario, Giuseppina De Luca, Monica Nardi, and Antonio Procopio. 2014. "An Erbium-Based Bifuctional Heterogeneous Catalyst: A Cooperative Route Towards C-C Bond Formation" Molecules 19, no. 7: 10218-10229. https://doi.org/10.3390/molecules190710218