Capsaicin: A Potent Inhibitor of Carbonic Anhydrase Isoenzymes
Abstract
:1. Introduction
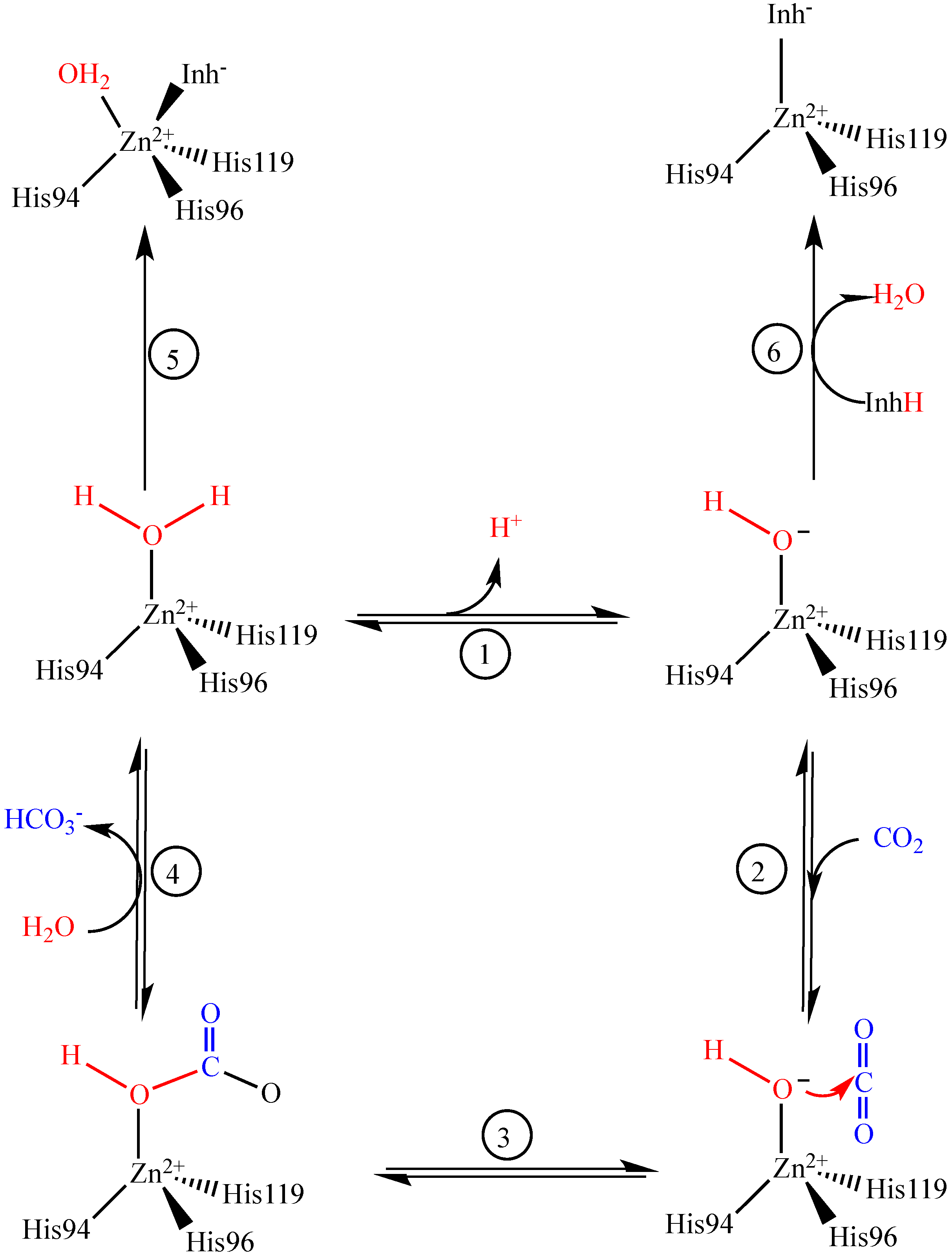
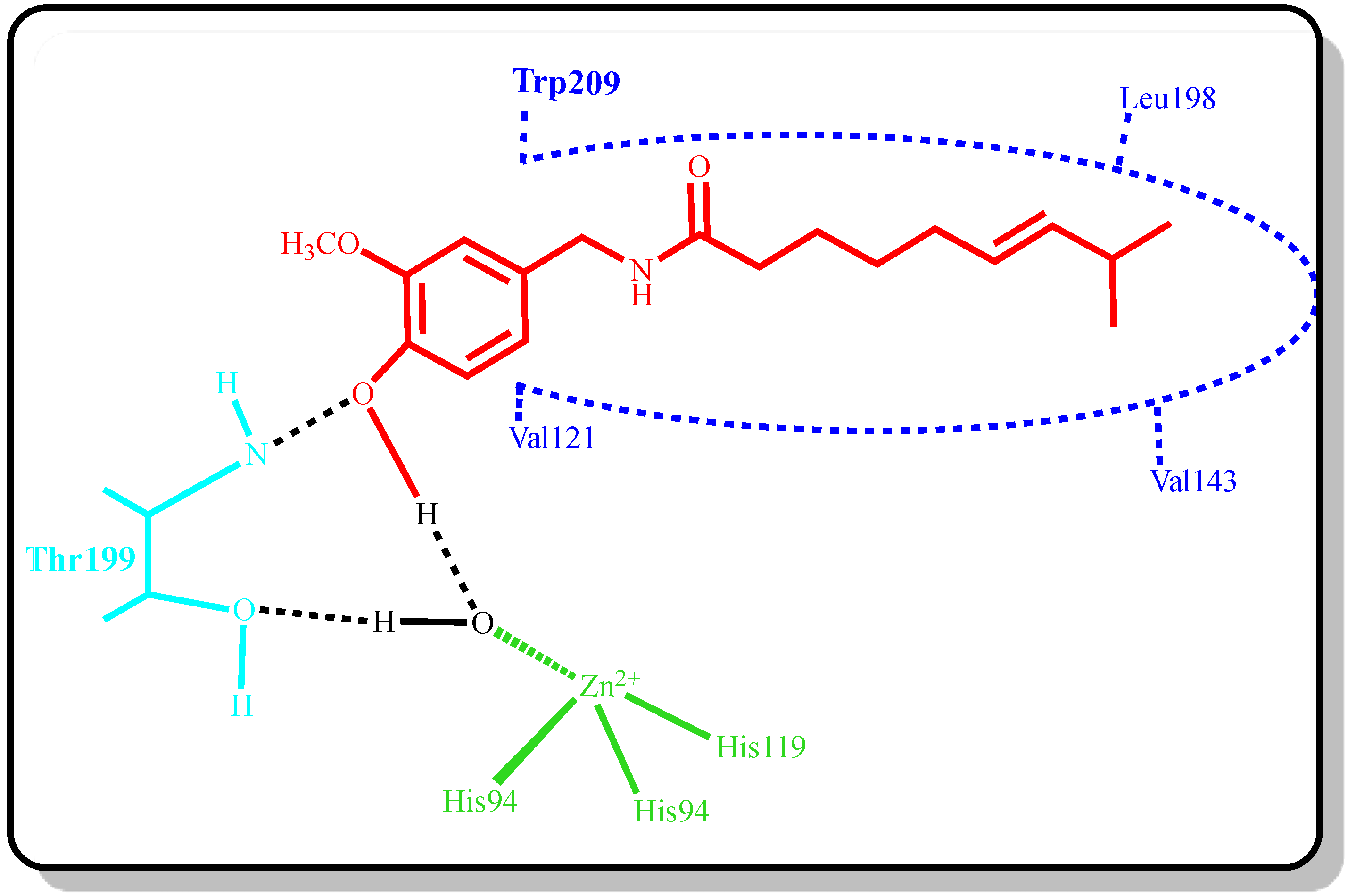
2. Results and Discussion

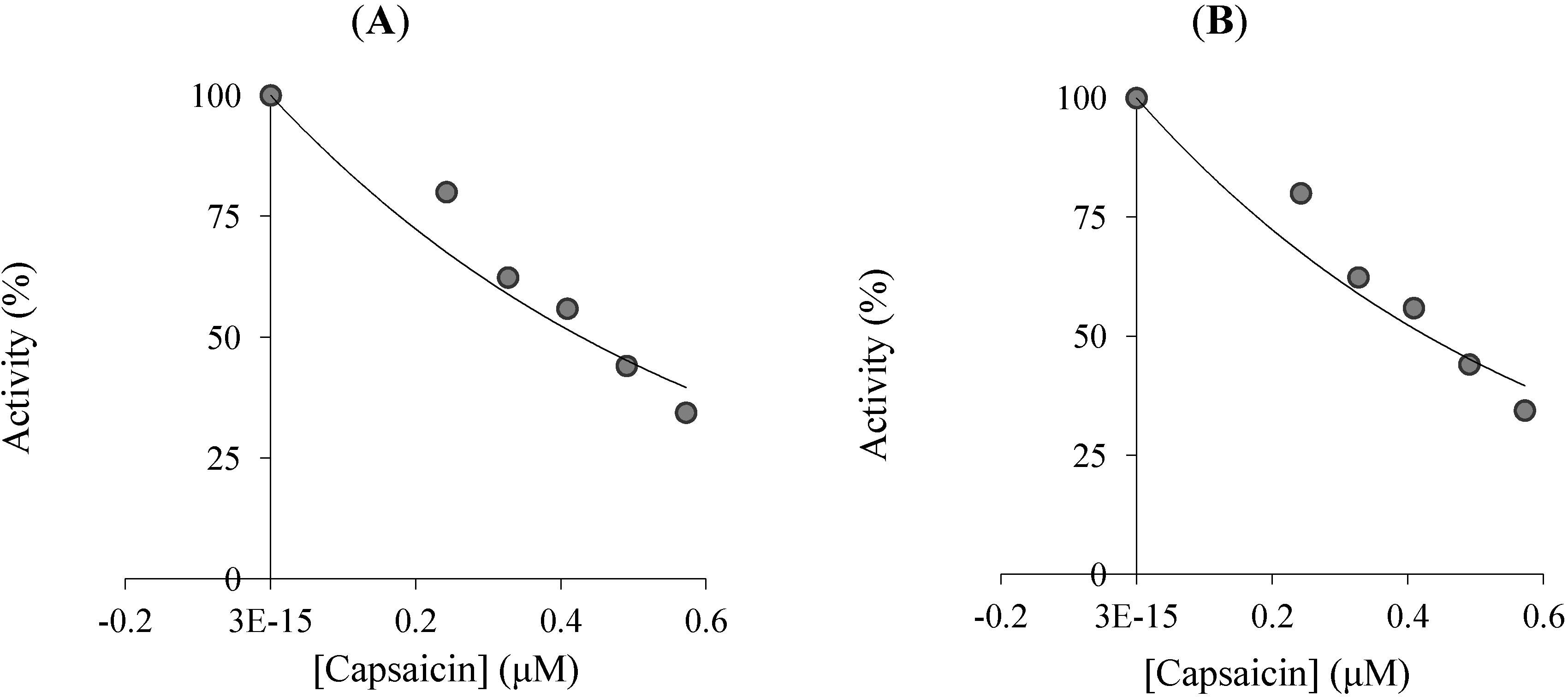
| Kinetic Parameters | hCA I | hCA II |
|---|---|---|
| IC50 (µM) | 428.04 | 316.01 |
| Ki (µM) | 696.15 ± 59.37 | 208.37 ± 14.38 |
| Inhibition type | Uncompetitive | Uncompetitive |
3. Experimental
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. 2007, 15, 4336–4350. [Google Scholar] [CrossRef]
- Żołnowska, B.; Sławiński, J.; Pogorzelska, A.; Chojnacki, J.; Vullo, D.; Supuran, C.T. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N'-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur. J. Med. Chem. 2014, 71, 135–147. [Google Scholar] [CrossRef]
- Aksu, K.; Nar, M.; Tanç, M.; Vullo, D.; Gülçin, İ.; Göksu, S.; Tümer, F.; Supuran, C.T. The synthesis of sulfamide analogues of dopamine related compounds and their carbonic anhydrase inhibitory properties. Bioorg. Med. Chem. 2013, 21, 2925–2931. [Google Scholar] [CrossRef]
- Akıncıoğlu, A.; Topal, M.; Gülçin, İ.; Göksu, S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch. Pharm. 2014, 347, 68–76. [Google Scholar] [CrossRef]
- Çetinkaya, Y.; Göçer, H.; Göksu, S.; Gülçin, İ. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of novel benzylamine derivatives. J. Enzyme Inhib. Med. Chem. 2014, 29, 168–174. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Disc. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Akbaba, Y.; Akıncıoğlu, A.; Göçer, H.; Göksu, S.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J. Enzyme Inhib. Med. Chem. 2014, 29, 35–42. [Google Scholar]
- Gülçin, İ.; Beydemir, Ş.; Büyükokuroğlu, M.E. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol. Pharm. Bull. 2004, 27, 613–616. [Google Scholar] [CrossRef]
- Beydemir, Ş.; Gülçin, İ. Effect of melatonin on carbonic anhydrase from human erythrocyte in vitro and from rat erythrocyte in vivo. J. Enzyme Inhib. Med. Chem. 2004, 19, 193–197. [Google Scholar]
- Gülçin, İ.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar]
- Guney, M.; Coskun, A.; Topal, F.; Dastan, A.; Gulcin, I.; Supuran, C.T. Oxidation of cyanobenzocycloheptatrienes: Synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg. Med. Chem. 2014, 22, 3537–3543. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef]
- Nar, M.; Çetinkaya, Y.; Gülçin, İ.; Menzek, A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J. Enzyme Inhib. Med. Chem. 2013, 28, 402–406. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrase inhibitors and their therapeutic potential. Exp. Opin. Ther. Patents 2000, 10, 575–600. [Google Scholar]
- Mincione, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma Carbonic Anhydrase Inhibitors as Ophthalomologic Drugs. In Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications; Supuran, C.T., Winum, J.Y., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 139–154. [Google Scholar]
- De Simone, G.; di Fiore, A.; Supuran, C.T. Are carbonic anhydrase inhibitors suitable for obtaining antiobesity drugs? Curr. Pharm. Des. 2008, 14, 655–660. [Google Scholar]
- Sethi, K.K.; Verma, S.M.; Tanç M.; Purper, G.; Calafato, G.; Carta, F.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of the human carbonic anhydrase isoforms I, II, IX and XII with benzene sulfonamides incorporating 4- and 3-nitrophthalimide moieties. Bioorg. Med. Chem. 2014, 22, 1586–1595. [Google Scholar] [CrossRef]
- Franchi, M.; Vullo, D.; Gallori, E.; Antel, J.; Wurl, M.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human and murine mitochondrial isozymes V with anions. Bioorg. Med. Chem. Lett. 2003, 13, 2857–2861. [Google Scholar]
- Banjia, D.; Banjia, O.J.F.; Reddy, M.; Annamalai, A.R. Impact of zinc, selenium and lycopene on capsaicin induced mutagenicity and oxidative damage in mice. J. Trace Elem. Med. Biol. 2013, 27, 230–235. [Google Scholar]
- Schwarz, N.A.; Spillane, M.; la Bounty, P.; Grandjean, P.W.; Leutholtz, B.; Willoughby, D.S. Capsaicin and evodiamine ingestion does not augment energy expenditure and fat oxidation at rest or after moderately-intense exercise. Nutr. Res. 2013, 33, 1034–1042. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, W.; Zhang, J.; Wang, M.; Firempong, C.K.; Feng, C.; Liu, H.; Xu, X.; Yu, J. Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: Preparation, in vitro and in vivo evaluation. J. Funct. Foods 2014, 8, 358–366. [Google Scholar]
- Contreras-Padilla, M.; Yahia, E.M. Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum-production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–473. [Google Scholar] [CrossRef]
- Surh, Y.J.; Lee, S.S. Capsaicin, a double-edged sword: Toxicity, metabolism, and chemopreventive potential. Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef]
- Chowdhury, B.; Mukhopadhyay, S.; Bhattacharayay, D.; De, A.K. Capsaicin, a unique anti-oxidant, anti-inflammatory, analgesic compound with antifungal activity against dermatophytes. Med. Sci. Res. 1996, 24, 669–670. [Google Scholar]
- Lee, J.G; Yon, J.M; Lin, C; Jung, A.Y.; Jung, K.Y.; Nam, S.Y. Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem. Toxicol. 2012, 50, 3877–3885. [Google Scholar] [CrossRef]
- Baboota, R.K.; Bishnoi, M.; Ambalam, P.; Kondepudi, K.K.; Sarma, S.M.; Boparai, R.K.; Podili, K. Functional food ingredients for the management of obesity and associated co-morbidities-A review. J. Funct. Foods 2013, 5, 997–1012. [Google Scholar]
- Joe, B.; Lokesh, B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta 1994, 1224, 255–263. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, J.G.; Yon, J.M.; Oh, K.W.; Baek, I.J.; Nahm, S.S.; Lee, B.J.; Yun, Y.W.; Nam, S.Y. Capsaicin prevents kainic acid-induced epileptogenesis in mice. Neurochem. Int. 2011, 58, 634–640. [Google Scholar]
- Kang, J.Y.; Alexander, B.; Math, M.V.; Williamson, R.C. The effect of chilli and its pungent ingredient capsaicin on gastrointestinal transit in the rat. J. Gastroenterol. Hepatol. 1993, 8, 513–516. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef]
- Sugimoto, T.; Xiao, C.; Ichikawa, H. Neonatal primary neuronal death induced by capsaicin and axotomy involves an apoptotic mechanism. Brain Res. 1998, 807, 147–154. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawada, T.; Yamamoto, M.; Iwai, K. Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem. Biophys. Res. Commun. 1987, 142, 259–264. [Google Scholar]
- Janssens, P.L.H.R.; Hursel, R.; Martens, E.A.P.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One 2013, 8, e67786. [Google Scholar]
- Yoshioka, M.; St-Pierre, S.; Suzuki, M.; Tremblay, A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br. J. Nutr. 1998, 80, 503–510. [Google Scholar]
- Topal, M.; Gülçin, İ. Rosmarinic acid: A potent carbonic anhydrase isoenzymes inhibitor. Turk. J. Chem. 2014. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol-a structure and activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar]
- Gülçin, İ. Antioxidant activity of food constituents-An overview. Arch. Toxicol. 2012, 86, 345–396. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Öztürk Sarikaya, S.B.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem. Biol. Drug Des. 2010, 75, 515–520. [Google Scholar] [CrossRef]
- Innocenti, A.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg. Med. Chem. Lett. 2008, 18, 1583–1587. [Google Scholar]
- Innocenti, A.; Öztürk Sarıkaya, S.B.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef]
- Innocenti, A.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of mammalian isoforms I–XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg. Med. Chem. 2008, 16, 7424–7428. [Google Scholar]
- Davis, R.A.; Innocenti, A.; Poulsen, S.A.; Supuran, C.T. Carbonic anhydrase inhibitors: Identification of selective inhibitors of the human mitochondrial isozymes VA and VB over the cytosolic isozymes I and II from a natural product-based phenolic library. Bioorg. Med. Chem. 2010, 18, 14–18. [Google Scholar] [CrossRef]
- Nair, S.K.; Ludwig, P.A.; Christianson, D.W. Phenol as a carbonic anhydrase inhibitor. J. Am. Chem. Soc. 1994, 116, 3659–3660. [Google Scholar] [CrossRef]
- Senturk, M.; Gulcin, I.; Dastan, A.; Kufrevioglu, O.I.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar]
- Ogilvie, J.M.; Ohlemiller, K.K.; Shah, G.N.; Ulmasov, B.; Becker, T.A.; Waheed, A.; Hennig, A.K.; Lukasiewicz, P.D.; Sly, W.S. Carbonic anhydrase XIV deficiency produces a functional defect in the retinal light response. Proc. Natl. Acad. Sci. USA 2007, 104, 8514–8519. [Google Scholar] [CrossRef]
- Avvaru, B.S.; Busby, S.A.; Chalmers, M.J.; Griffin, P.R.; Venkatakrishnan, B.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. Apo-human carbonic anhydrase II revisited: Implications of the loss of a metal in protein structure, stability, and solvent network. Biochemistry 2009, 48, 7365–7372. [Google Scholar] [CrossRef]
- Göçer, H.; Gülçin, İ. Caffeic acid phenethyl ester (CAPE): A potent carbonic anhydrase isoenzymes inhibitor. Int. J. Acad. Res. 2013, 5, 150–155. [Google Scholar] [CrossRef]
- Çoban, T.A.; Beydemir, Ş.; Gülçin, İ.; Ekinci, D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol. Pharm. Bull. 2007, 30, 2257–2261. [Google Scholar] [CrossRef]
- ArasHisar, Ş.; Hisar, O.; Beydemir, Ş.; Gülçin, İ.; Yanık, T. Effect of vitamin E on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Acta Vet. Hung. 2004, 52, 413–422. [Google Scholar] [CrossRef]
- Balaydın, H.T.; Gülçin, İ.; Menzek, A.; Göksu, S.; Şahin, E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J. Enzyme Inhib. Med. Chem. 2010, 25, 685–695. [Google Scholar] [CrossRef]
- Akıncıoğlu, A.; Akbaba, Y.; Göçer, H.; Göksu, S.; Gülçin, İ.; Supuran, C.T. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg. Med. Chem. 2013, 21, 379–1385. [Google Scholar] [CrossRef]
- Çetinkaya, Y.; Göçer, H.; Gülçin, İ.; Menzek, A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch. Pharm. 2014, 347, 354–359. [Google Scholar] [CrossRef]
- Hisar, O.; Beydemir, Ş.; Gülçin, İ.; Küfrevioğlu, Ö.İ.; Supuran, C.T. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhyncytes mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J. Enzyme Inhib. Med. Chem. 2005, 20, 35–39. [Google Scholar] [CrossRef]
- Laemmli, D.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–683. [Google Scholar] [CrossRef]
- Gülçin, İ.; Küfrevioğlu, Ö.İ.; Oktay, M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibition effects of some chemicals on the enzyme activity. J. Enzyme Inhib. Med. Chem. 2005, 20, 297–302. [Google Scholar] [CrossRef]
- Beydemir, Ş.; Gülçin, İ.; Hisar, O.; Küfrevioğlu, Ö.İ.; Yanık, T. Effect of melatonin on glucose-6-phospate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. J. Appl. Anim. Res. 2005, 28, 65–68. [Google Scholar] [CrossRef]
- Çoban, T.A.; Beydemir, Ş.; Gülçin, İ.; Ekinci, D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: In vivo and in vitro studies. J. Enzyme Inhib. Med. Chem. 2008, 23, 266–270. [Google Scholar] [CrossRef]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [Google Scholar]
- Şentürk, M.; Gülçin, İ.; Daştan A.; Küfrevioğlu, Ö.İ.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar]
- Göçer, H.; Akıncıoğlu, A.; Öztaşkın, N.; Göksu, S.; Gülçin, İ. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch. Pharm. 2013, 346, 783–792. [Google Scholar] [CrossRef]
- Coban, T.A.; Beydemir, S.; Gücin, İ.; Ekinci, D.; Innocenti, A.; Vullo, D.; Supuran, C.T. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg. Med. Chem. 2009, 17, 5791–5795. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–251. [Google Scholar]
- Köksal, E.; Gülçin, İ. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L.) buds. Protein Peptide Lett. 2008, 15, 320–326. [Google Scholar] [CrossRef]
- Şentürk, M.; Gülçin, İ.; Çiftci, M.; Küfrevioğlu, Ö.İ. Dantrolene inhibits human erythrocyte glutathione reductase. Biol. Pharm. Bull. 2008, 31, 2036–2039. [Google Scholar]
- Iga, D.P.; Schmidt, R.; Iga, S.; Hotoleanu, C.L.; Duica, F.; Nicolescu, A.; Gitman, S.S. Synthesis and characterization of new chromogenic substrates for exoglycosidases: a-Glucosidase, a-mannosidase, andβ-galactosidase. Turk. J. Chem. 2013, 37, 299–307. [Google Scholar]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar]
- Çetinkaya, Y.; Göçer, H.; Menzek, A.; Gülçin, İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef]
- Innocenti, A.; Gülçin, İ.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg. Med. Chem. Lett. 2010, 20, 5050–5053. [Google Scholar]
- Öztürk Sarıkaya, S.B.; Topal, F.; Şentürk, M.; Gülçin, İ.; Supuran, C.T. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg. Med. Chem. Lett. 2011, 21, 4259–4262. [Google Scholar] [CrossRef]
- Balaydın, H.T.; Senturk, M.; Goksu, S.; Menzek, A. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols and their derivatives including natural products: Vidalol B. Eur. J. Med. Chem. 2012, 54, 423–428. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A Potent Inhibitor of Carbonic Anhydrase Isoenzymes. Molecules 2014, 19, 10103-10114. https://doi.org/10.3390/molecules190710103
Arabaci B, Gulcin I, Alwasel S. Capsaicin: A Potent Inhibitor of Carbonic Anhydrase Isoenzymes. Molecules. 2014; 19(7):10103-10114. https://doi.org/10.3390/molecules190710103
Chicago/Turabian StyleArabaci, Betul, Ilhami Gulcin, and Saleh Alwasel. 2014. "Capsaicin: A Potent Inhibitor of Carbonic Anhydrase Isoenzymes" Molecules 19, no. 7: 10103-10114. https://doi.org/10.3390/molecules190710103







