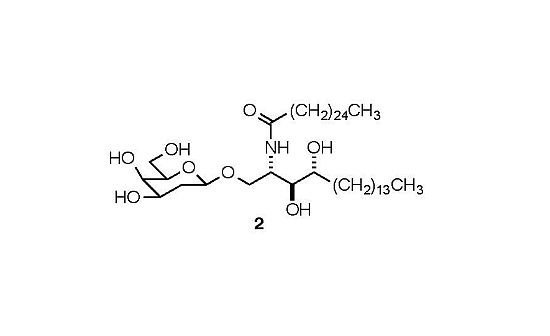
Synthesis of a 2ꞌꞌ-Deoxy-β-GalCer
Abstract
:1. Introduction
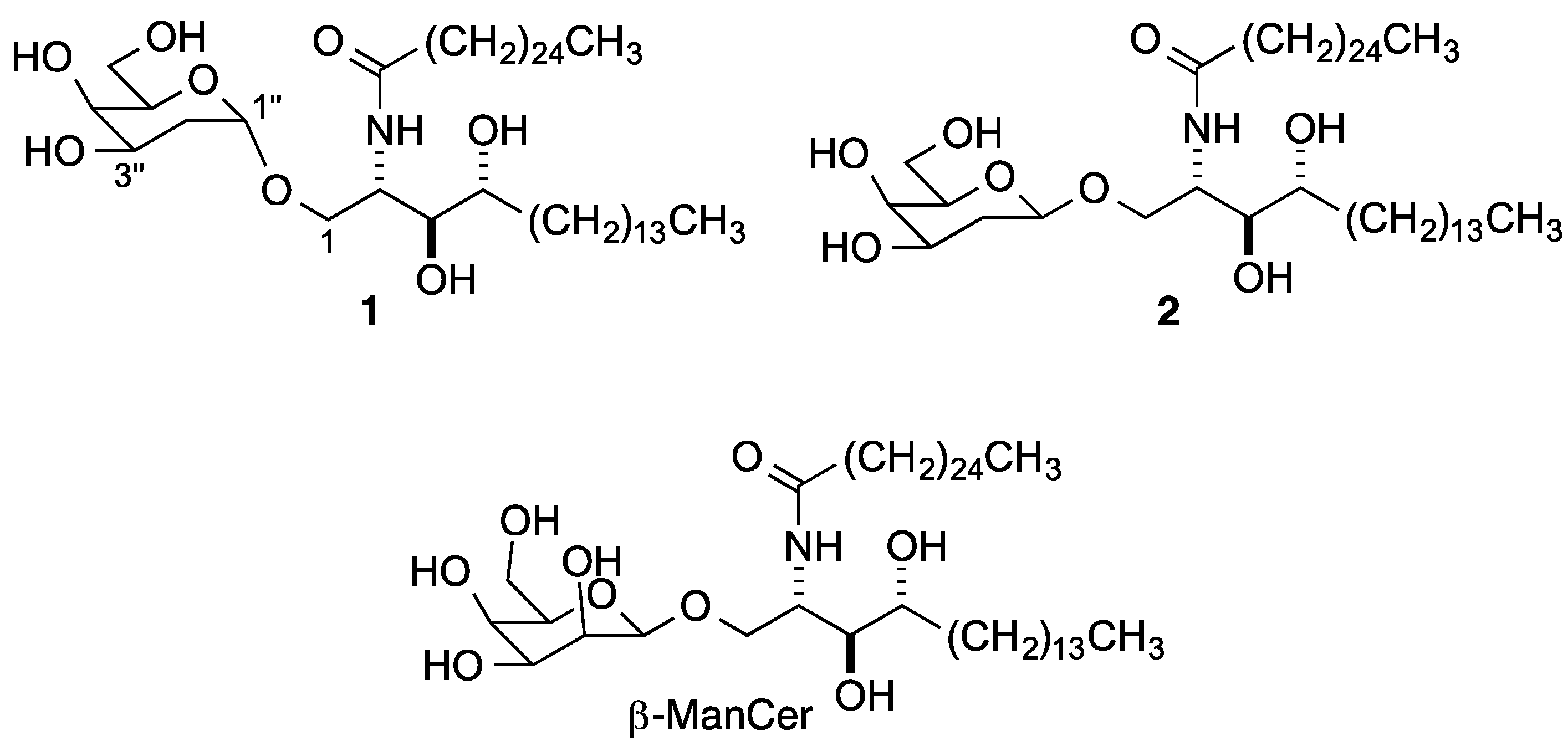
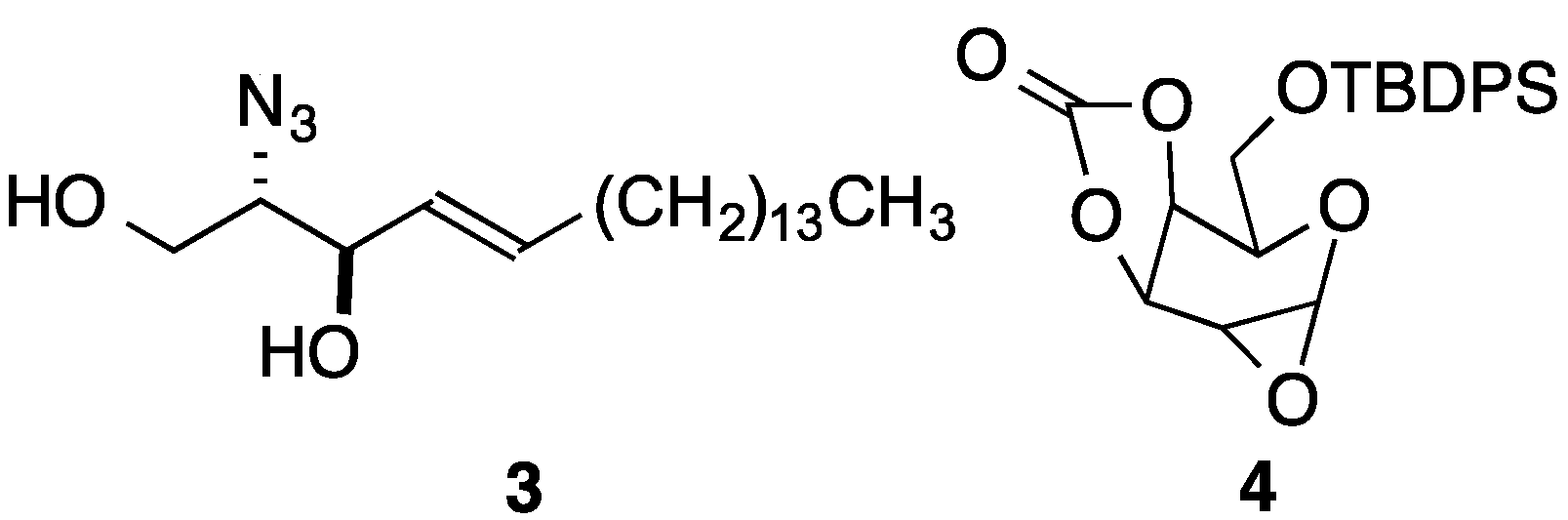
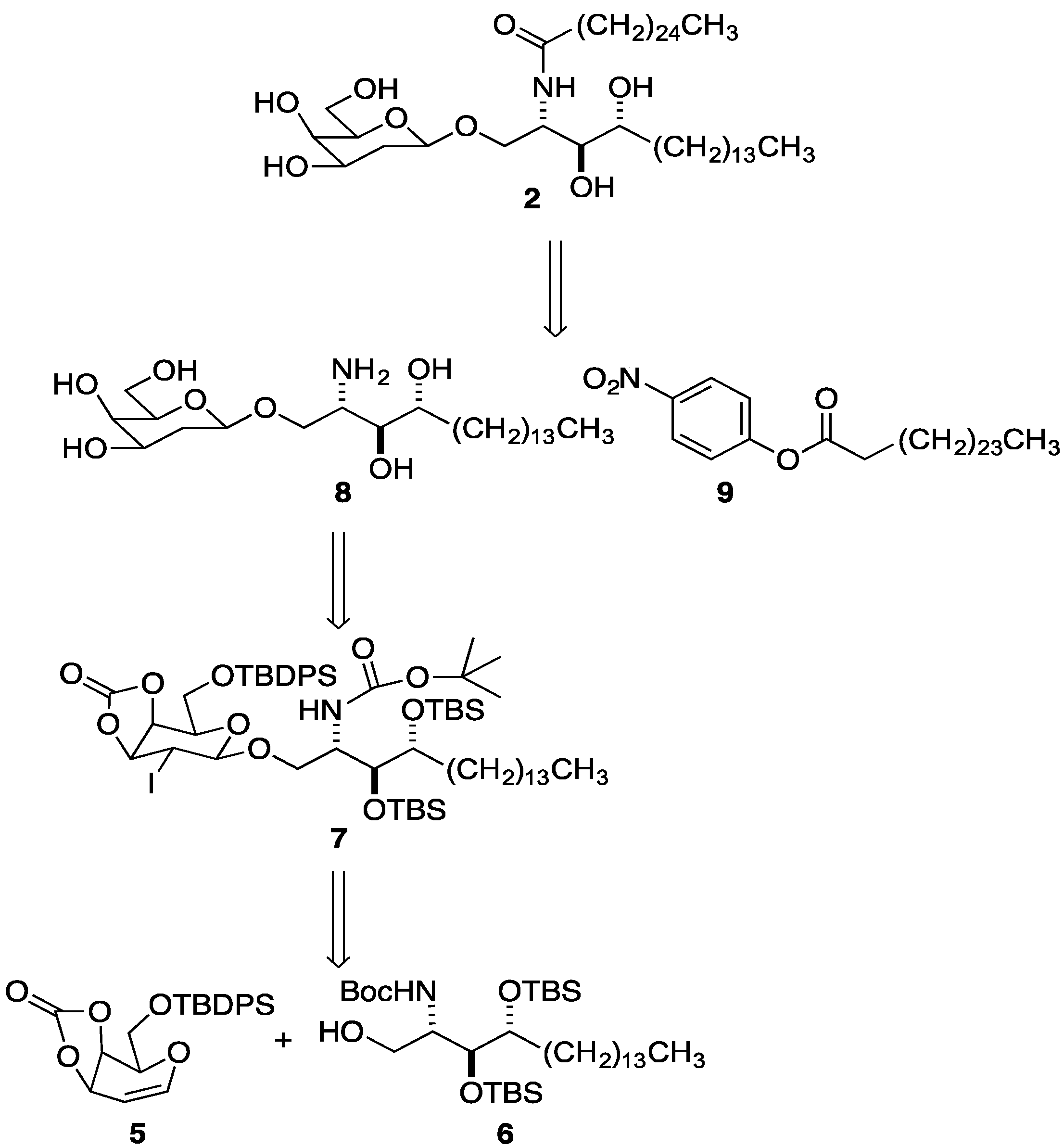
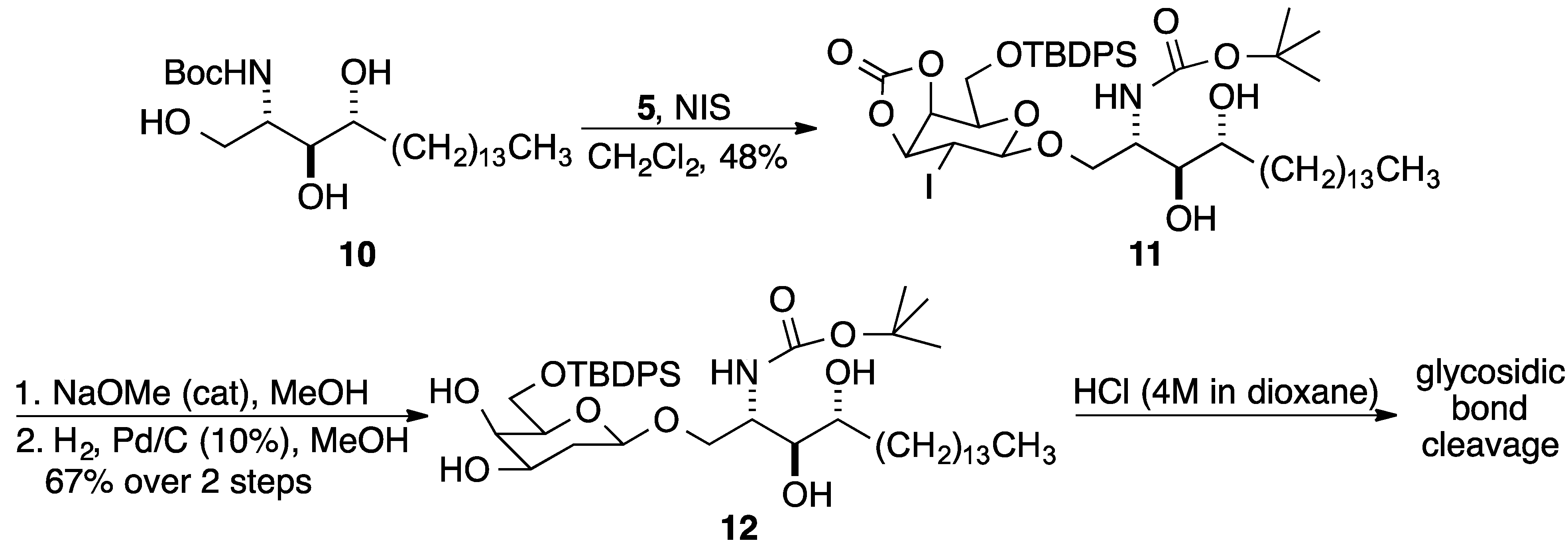
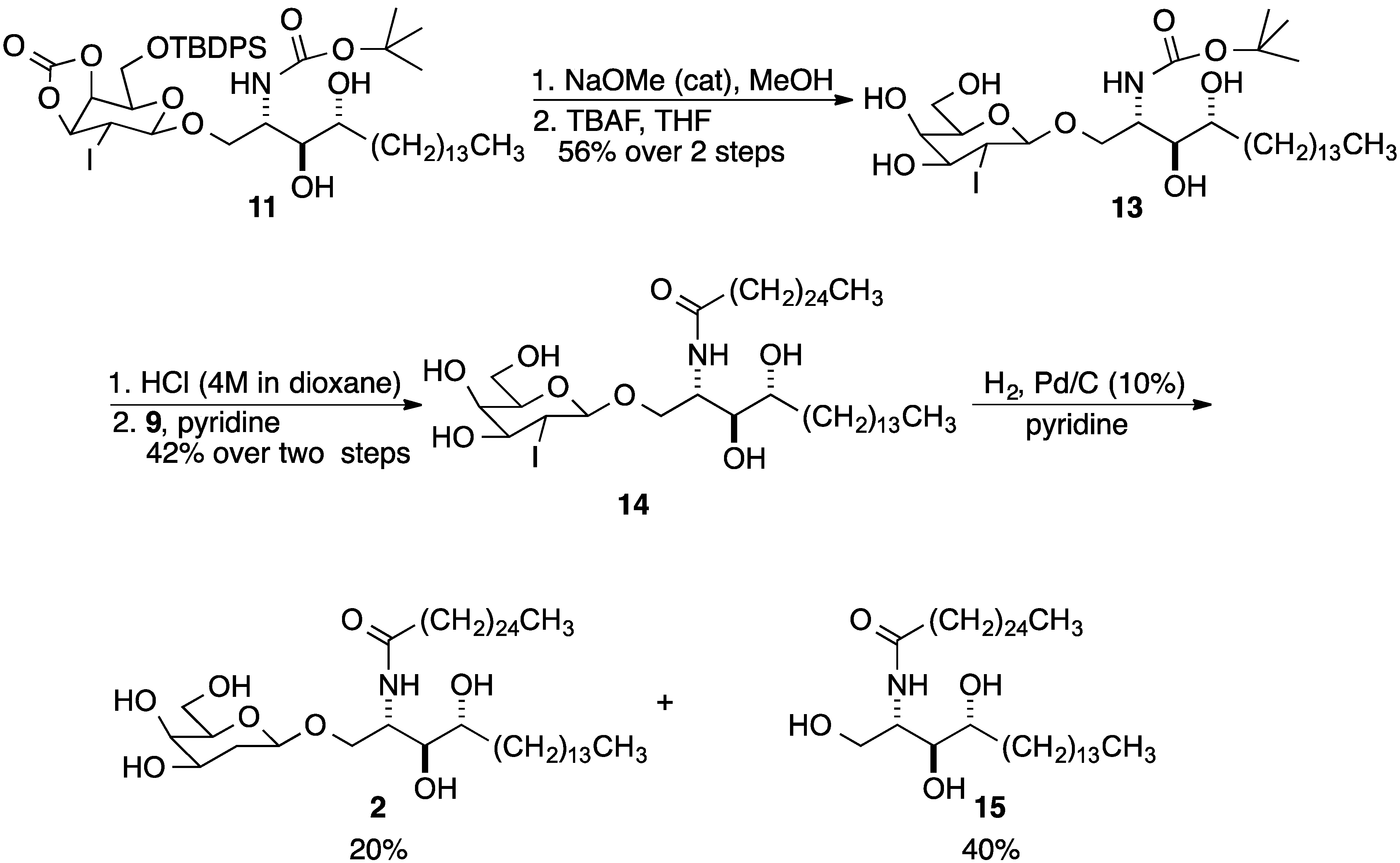
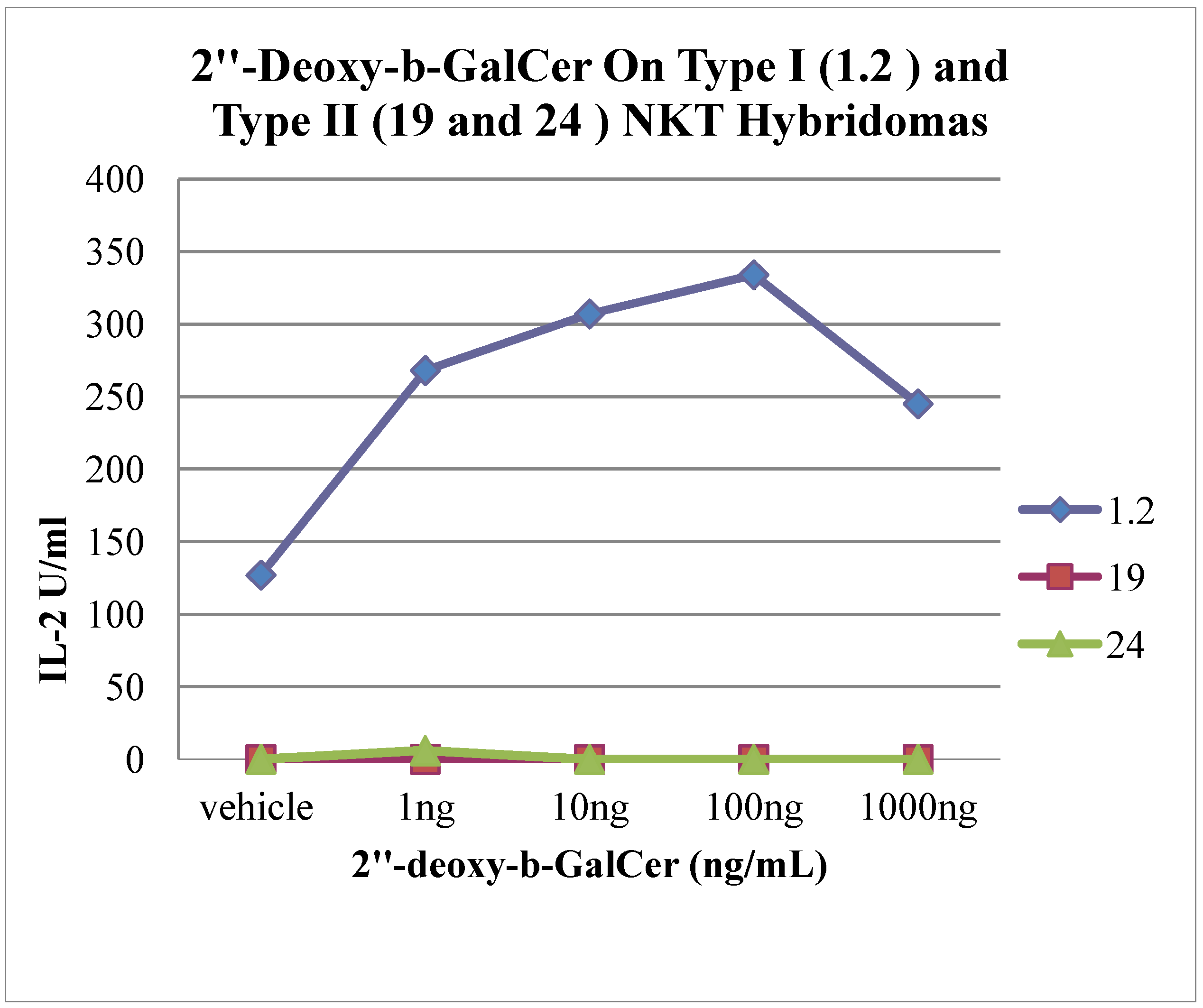
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. Biological Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-restricted Antigens by Natural Killer T Cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef]
- Adams, E.J.; Luoma, A.M. The yin and yang of CD1d recognition. Nat. Immunol. 2012, 13, 814–815. [Google Scholar] [CrossRef]
- Girardi, E.; Zajonc, D.M. Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol. Rev. 2012, 250, 167–179. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Pellicci, D.G.; Patel, O.; Kjer-Nielsen, L.; McCluskey, J.; Rossjohn, J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin. Immunol. 2010, 22, 61–67. [Google Scholar] [CrossRef]
- Zajonc, D.M.; Kronenberg, M. Carbohydrate specificity of the recognition of diverse glycolipids by natural killer T cells. Immunol. Rev. 2009, 230, 188–200. [Google Scholar] [CrossRef]
- Lawson, V. Turned on by danger: Activation of CD1d-restricted invariant natural killer T cells. J. Immunol. 2012, 137, 20–27. [Google Scholar] [CrossRef]
- Tashiro, T. Structure-activity relationship studies of novel glycosphingolipids that stimulate natural killer T-cells. Biosci. Biotech. Bioch. 2012, 76, 1055–1067. [Google Scholar] [CrossRef]
- Mallevaey, T.; Selvanantham, T. Strategy of lipid recognition by invariant natural killer T cells: ‘One for all and all for one’. Immunology 2012, 136, 273–282. [Google Scholar] [CrossRef]
- Banchet-Cadeddu, A.; Henon, E.; Dauchez, M.; Renault, J.-H.; Monneaux, F.; Haudrechy, A. The stimlating adventure of KRN7000. Org. Biomol. Chem. 2011, 9, 3080–3104. [Google Scholar] [CrossRef]
- Venkataswamya, M.M.; Porcelli, S.A. Lipid and glycolipid antigens of CD1D-restricted natural killer T cells. Semin. Immunol. 2010, 22, 68–78. [Google Scholar]
- Laurent, X.; Bertin, B.; Renault, N.; Farce, A.; Speca, S.; Milhomme, O.; Millet, R.; Desreumaux, P.; Henon, E.; Chavatte, P. Switching invariant natural killer t (inkt) cell response from anticancerous to anti-inflammatory effect: Molecular bases. J. Med. Chem. 2014. In Print. [Google Scholar]
- Izhak, L.; Ambrosino, E.; Kato, S.; Parish, S.T.; O’Konek, J.J.; Weber, H.; Xia, Z.; Venzon, D.; Berzofsky, J.A.; Terabe, M. Delicate balance among three types of T cells in concurrent regulation of tumor immunity. Cancer Res. 2013, 73, 1514–1523. [Google Scholar] [CrossRef]
- Viale, R.; Ware, R.; Maricic, I.; Chaturvedi, V.; Kumar, V. NKT cell subsets can exert opposing effects in autoimmunity, tumor surveillance and inflammation. Curr. Immunol. Rev. 2012, 8, 287–296. [Google Scholar] [CrossRef]
- Rhost, S.; Sedimbi, S.; Kadri, N.; Cardell, S.L. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand. J. Immunol. 2012, 76, 246–255. [Google Scholar] [CrossRef]
- Arrenberg, P.; Maricic, I.; Kumar, V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology 2011, 140, 646–655. [Google Scholar]
- Sundell, I.B.; Halder, R.; Zhang, M.; Maricic, I.; Koka, P.S.; Kumar, V. Sulfatide administration leads to inhibition of HIV-1 replication and enhanced hematopoeisis. J. Stem Cells 2010, 5, 33–42. [Google Scholar]
- Berzofsky, J.A.; Terabe, M. The contrasting roles of NKT cells in tumor immunity. Curr. Mol. Med. 2009, 9, 667–672. [Google Scholar] [CrossRef]
- Wu, D.; Fujio, M.; Wong, C.-H. Glycolipids as immunostimulating agents. Bioorg. Med. Chem. 2008, 16, 1073–1083. [Google Scholar]
- Savage, P.A.; Teyton, L.; Bendelac, A. Glycolipids for natural killer T cells. Chem. Soc. Rev. 2006, 35, 771–779. [Google Scholar] [CrossRef]
- Tyznik, A.J.; Farber, E.; Girardi, E.; Birkholz, A.; Li, Y.; Chitale, S.; So, R.; Arora, P.; Khurana, A.; Wang, J.; et al. Novel glycolipids that elicit IFN-γ responses from natural killer T cells. Chem. Biol. 2011, 18, 1620–1630. [Google Scholar] [CrossRef]
- Wu, D.; Zajonc, D.M.; Fujio, M.; Sullivan, B.; Kinjo, Y.; Kronenberg, M.; Wilson, I.A.; Wong, C.-H. Design of natural killer T cell activators: Structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc. Natl. Acad. Sci. USA 2006, 103, 3972–3977. [Google Scholar]
- Koch, M.; Stronge, V.S.; Shepherd, D.; Gadola, S.D.; Matthew, B.; Ritter, G.; Fersht, A.R.; Besra, G.S.; Schmidt, R.R.; Jones, E.Y.; et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat. Immunol. 2005, 6, 819–826. [Google Scholar]
- Wun, K.S.; Cameron, G.; Patel, O.; Pang, S.S.; Pellicci, D.G.; Sullivan, L.C.; Keshipeddy, S.; Young, M.H.; Uldrich, A.P.; Thakur, M.S.; et al. A molecular basis for the exquisite CD1d-restricted antigen-specificity and functional responses of natural killer T cells. Immunity 2011, 34, 327–339. [Google Scholar] [CrossRef]
- Wun, K.S.; Ross, F.; Patel, O.; Besra, G.S.; Porcelli, S.A.; Richardson, S.K.; Keshipeddy, S.; Howell, A.R.; Godfrey, D.I.; Rossjohn, J. Human and mouse type I natural killer T cell antigen receptors exhibit different fine specificities for CD1d-antigen complex. J. Biol. Chem. 2012, 287, 39139–39148. [Google Scholar] [CrossRef]
- Zajonc, D.M.; Savage, P.B.; Bendelac, A.; Wilson, I.A.; Teyton, L. Crystal structures of mouse CD1d-iGb3 complex and its cognate V.alpha.14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J. Mol. Biol. 2008, 377, 1104–1116. [Google Scholar] [CrossRef]
- Wun, K.S.; Borg, N.A.; Kjer-Nielsen, L.; Beddoe, T.; Koh, R.; Richardson, S.K.; Thakur, M.; Howell, A.R.; Scott-Browne, J.P.; Gapin, L.; et al. A minimal binding footprint on CD1d glycolipid is a basis for selection of the unique human NKT TCR. J. Exp. Med. 2008, 205, 939–949. [Google Scholar] [CrossRef]
- Scott-Browne, J.P.; Matsuda, J.L.; Mallevaey, T.; White, J.; Borg, N.A.; McCluskey, J.; Rossjohn, J.; Kappler, J.; Marrack, P.; Gapin, L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat. Immunol. 2007, 8, 1105–1113. [Google Scholar] [CrossRef]
- Borg, N.A.; Wun, K.S.; Kjer-Nielsen, L.; Wilce, M.C.J.; Pellicci, D.G.; Koh, R.; Besra, G.S.; Bharadwaj, M.; Godfrey, D.I.; McCluskey, J.; et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 2007, 448, 44–49. [Google Scholar] [CrossRef]
- Girardi, E.; Maricic, I.; Wang, J.; Mac, T.-T.; Iyer, P.; Kumar, V.; Zajonc, D.M. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat. Immunol. 2012, 13, 851–856. [Google Scholar] [CrossRef]
- Patel, O.; Pellicci, D.G.; Gras, S.; Sandoval-Romero, M.L.; Uldrich, A.P.; Mallevaey, T.; Clarke, A.J.; le Nours, J.; Theodossis, A.; Cardell, S.L.; et al. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat. Immunol. 2012, 13, 857–863. [Google Scholar] [CrossRef]
- Zajonc, D.M.; Maricic, I.; Wu, D.; Halder, R.; Keshab, R.; Wong, C.-H.; Kumar, V.; Wilson, I.A. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J. Exp. Med. 2005, 202, 1517–1526. [Google Scholar] [CrossRef]
- Iijima, H.; Kimura, K.; Sakai, T.; Uchimura, A.; Shimizu, T.; Ueno, H.; Natori, T.; Koezuka, Y. Structure-activity relationship and conformational analysis of monoglycosylceramides on the syngeneic mixed leukocyte reaction. Bioorg. Med. Chem. 1998, 6, 1905–1910. [Google Scholar] [CrossRef]
- Brennan, P.J.; Tatituri, R. V.V.; Brigl, M.; Kim, E.Y.; Tuli, A.; Sanderson, J.P.; Gadola, S.D.; Hsu, F.-F.; Besra, G.S.; Brenner, M.B. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 2011, 12, 1202–1211. [Google Scholar] [CrossRef]
- O’Konek, J.J.; Illarionov, P.; Khursigara, D.S.; Ambrosino, E.; Izhak, L.; Castillo, B.F.I.; Raju, R.; Khalili, M.; Kim, H.-Y.; Howell, A.R.; et al. Mouse and human iNKT cell agonist β-mannosylceramide reveals a distinct mechanism of tumor immunity. J. Clin. Invest. 2011, 121, 683–694. [Google Scholar] [CrossRef]
- Toshima, K.; Tatsuta, K. Recent progress in O-glycosylation methods and its application to natural products synthesis. Chem. Rev. 1993, 93, 1503–1531. [Google Scholar]
- Takiura, K.; Honda, S. Hydroxy- and acetoxymercuration of D-glucal triacetate. Carbohyd. Res. 1972, 23, 369–377. [Google Scholar]
- Monneret, C.; Choay, P. A convenient synthesis of 2-deoxy-D-arabinohexose and its methyl and benzyl glycosides. Carbohyd. Res. 1981, 96, 299–305. [Google Scholar] [CrossRef]
- Giese, B.; Kopping, B.; Chatgilialoglu, C. Tris(trimethylsilyl)silane as mediator in organic synthesis via radicals. Tetrahedron Lett. 1989, 30, 681–684. [Google Scholar] [CrossRef]
- Thiem, J.; Klaffke, W. Syntheses of deoxyoligosaccharides. Top. Curr. Chem. 1990, 154, 285–332. [Google Scholar] [CrossRef]
- Gervay, J.; Danishefsky, S. A stereospecific route to 2-deoxy-β-glycosides. J. Org. Chem. 1991, 56, 5448–5451. [Google Scholar] [CrossRef]
- Durham, T.B.; Roush, W.R. Stereoselective synthesis of 2-deoxy-β-galactosides via 2-deoxy-2-bromo- and 2-deoxy-2-iodo-galactopyranosyl donors. Org. Lett. 2003, 5, 1871–1874. [Google Scholar] [CrossRef]
- Roush, W.R.; Bennett, C.E. A highly stereoselective synthesis of 2-deoxy-β-glycosides using 2-deoxy-2-iodo-glucopyranosyl acetate donors. J. Am. Chem. Soc. 1999, 121, 3541–3542. [Google Scholar] [CrossRef]
- Roush, W.R.; Briner, K.; Sebesta, D.P. Highly stereoselective synthesis of α-L-olivomycosides via trimethylsilyl triflate mediated glycosidations of 1-O-Acetyl-4-O-isobutyryl-2,6-dideoxy-2-iodo-3-C-methyl-α-L-mannopyranose. Synlett 1993, 264–266. [Google Scholar] [CrossRef]
- Roush, W.R.; Narayan, S. 2-Deoxy-2-iodo-α-mannopyranosyl and -talopyranosyl acetates: Highly stereoselective glycosyl donors for the synthesis of 2-deoxy-α-glycosides. Org. Lett. 1999, 1, 899–902. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Ladduwahetty, T.; Randall, J.L.; Chucholowski, A. Stereospecific 1,2-migrations in carbohydrates. Stereocontrolled synthesis of α- and β-2-deoxyglycosides. J. Am. Chem. Soc. 1986, 108, 2466–2467. [Google Scholar] [CrossRef]
- Pongdee, R.; Wu, B.; Sulikowski, G.A. One-pot synthesis of 2-deoxy-β-oligosaccharides. Org. Lett. 2001, 3, 3523–3525. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yanagiya, Y.; Honda, T.; Ikegami, S. A stereocontrolled construction of 2-deoxy-β-glycosidic linkages via 1,2-trans-β-glycosidation of 2-deoxy-2-[(p-methoxyphenyl)thio]glycopyranosyl N,N,N',N'-tetramethylphosphoroamidates. Chem. Lett. 1992, 1511–1514. [Google Scholar]
- Binkley, R.W.; Koholic, D.J. Photoremovable hydroxyl group protection. Use of the p-tolylsulfonyl protecting group in β-disaccharide synthesis. J. Org. Chem. 1989, 54, 3577–3581. [Google Scholar] [CrossRef]
- Toshima, K.; Misawa, M.; Ohta, K.; Tatsuta, K.; Kinoshita, M. Enantiospecific synthesis of C20-C28 segment of concanamycin a: Application of diethylisopropylsilyl protecting group. Tetrahedron Lett. 1989, 30, 6417–6420. [Google Scholar] [CrossRef]
- Hadd, M.J.; Gervay, J. Glycosyl iodides are highly efficient donors under neutral conditions. Carbohyd. Res. 1999, 320, 61–69. [Google Scholar] [CrossRef]
- Zhou, M.; O’Doherty, G.A. De novo approach to 2-deoxy-β-glycosides: Asymmetric syntheses of digoxose and digitoxin. J. Org. Chem. 2007, 72, 2485–2493. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshizawa, A.; Takahashi, T. Direct and stereoselective synthesis of β-linked 2,6-deoxyoligosaccharides. Angew. Chem., Int. Ed. 2007, 46, 2505–2507. [Google Scholar] [CrossRef]
- Morris, W.J.; Shair, M.D. Stereoselective synthesis of 2-deoxy-β-glycosides using anomeric O-alkylation/arylation. Org. Lett. 2009, 11, 9–12. [Google Scholar] [CrossRef]
- Gervay, J.; Peterson, J.M.; Oriyama, T.; Danishefsky, S.J. An unexpected sialylation: Total syntheses of ganglioside GM4 and a positional isomer. J. Org. Chem. 1993, 58, 5465–5468. [Google Scholar] [CrossRef]
- Zhang, Z.; Magnusson, G. Synthesis of double-chain bis-sulfone neoglycolipids of the 2'-, 3'- and 6'-deoxyglobotrioses. J. Org. Chem. 1995, 60, 7304–7305. [Google Scholar] [CrossRef]
- Randolph, J.T.; Danishefsky, S.J. First synthesis of a digitalis saponin. demonstration of the scope and limitations of a convergent scheme for branched oligosaccharide synthesis by the logic of glycal assembly. J. Am. Chem. Soc. 1995, 117, 5693–5700. [Google Scholar] [CrossRef]
- Blauvelt, M.L.; Khalili, M.; Jaung, W.; Paulsen, J.; Anderson, A.C.; Wilson, S.B.; Howell, A.R. α-S-Galcer: Synthesis and evaluation for iNKT cell stimulation. Bioorg. Med. Chem. Lett. 2008, 18, 6374–6376. [Google Scholar] [CrossRef]
- Burdin, N.; Brossay, L.; Degano, M.; Iijima, H.; Gui, M.; Wilson, I.A.; Kronenberg, M. Structural requirements for antigen presentation by mouse CD1. Proc. Natl. Acad. Sci. USA 2000, 97, 10156–10161. [Google Scholar] [CrossRef]
- Blomqvist, M.; Rhost, S.; Teneberg, S.; Loefbom, L.; Oesterbye, T.; Brigl, M.; Mansson, J.-E.; Cardell, S. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur. J. Immunol. 2009, 39, 1726–1735. [Google Scholar] [CrossRef]
- Naidenko, O.V.; Maher, J.K.; Ernst, W.A.; Sakai, T.; Modlin, R.L.; Kronenberg, M. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 1999, 190, 1069–1079. [Google Scholar] [CrossRef]
- Giabbai, B.; Sidobre, S.; Crispin, M.D.; Sanchez-Ruiz, Y.; Bachi, A.; Kronenberg, M.; Wilson, I.A.; Degano, M. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: A molecular basis for NKT cell activation. J. Immunol. 2005, 175, 977–984. [Google Scholar] [CrossRef]
- Jo, S.Y.; Kim, H.C.; Woo, S.W.; Seo, M.J.; Lee, G.; Kim, H.R. Synthesis of 1-substituted-phytosphingosine: Novel protection of phytosphingosine. Bull. Korean Chem. Soc. 2003, 24, 267–268. [Google Scholar] [CrossRef]
- Sidobre, S.; Hammond, K.J.L.; Benazet-Sidobre, L.; Maltsev, S.D.; Richardson, S.K.; Ndonye, R.; Howell, A.R.; Sakai, T.; Besra, G.S.; Porcelli, S.A.; et al. The TCR expressed by Vα14i NKT has a unique mode of antigen recognition. Proc. Natl. Acad. Sci. USA 2004, 101, 12254–12259. [Google Scholar] [CrossRef]
- Tupin, E.; Kronenberg, M. Activation and natural killer T cells by glycolipids. Methods Enzymol. 2006, 417, 185–201. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compound 2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, M.S.; Khurana, A.; Kronenberg, M.; Howell, A.R. Synthesis of a 2ꞌꞌ-Deoxy-β-GalCer. Molecules 2014, 19, 10090-10102. https://doi.org/10.3390/molecules190710090
Thakur MS, Khurana A, Kronenberg M, Howell AR. Synthesis of a 2ꞌꞌ-Deoxy-β-GalCer. Molecules. 2014; 19(7):10090-10102. https://doi.org/10.3390/molecules190710090
Chicago/Turabian StyleThakur, Meena S., Archana Khurana, Mitchell Kronenberg, and Amy R. Howell. 2014. "Synthesis of a 2ꞌꞌ-Deoxy-β-GalCer" Molecules 19, no. 7: 10090-10102. https://doi.org/10.3390/molecules190710090