Biological Activities and Phytochemical Profiles of Extracts from Different Parts of Bamboo (Phyllostachys pubescens)
Abstract
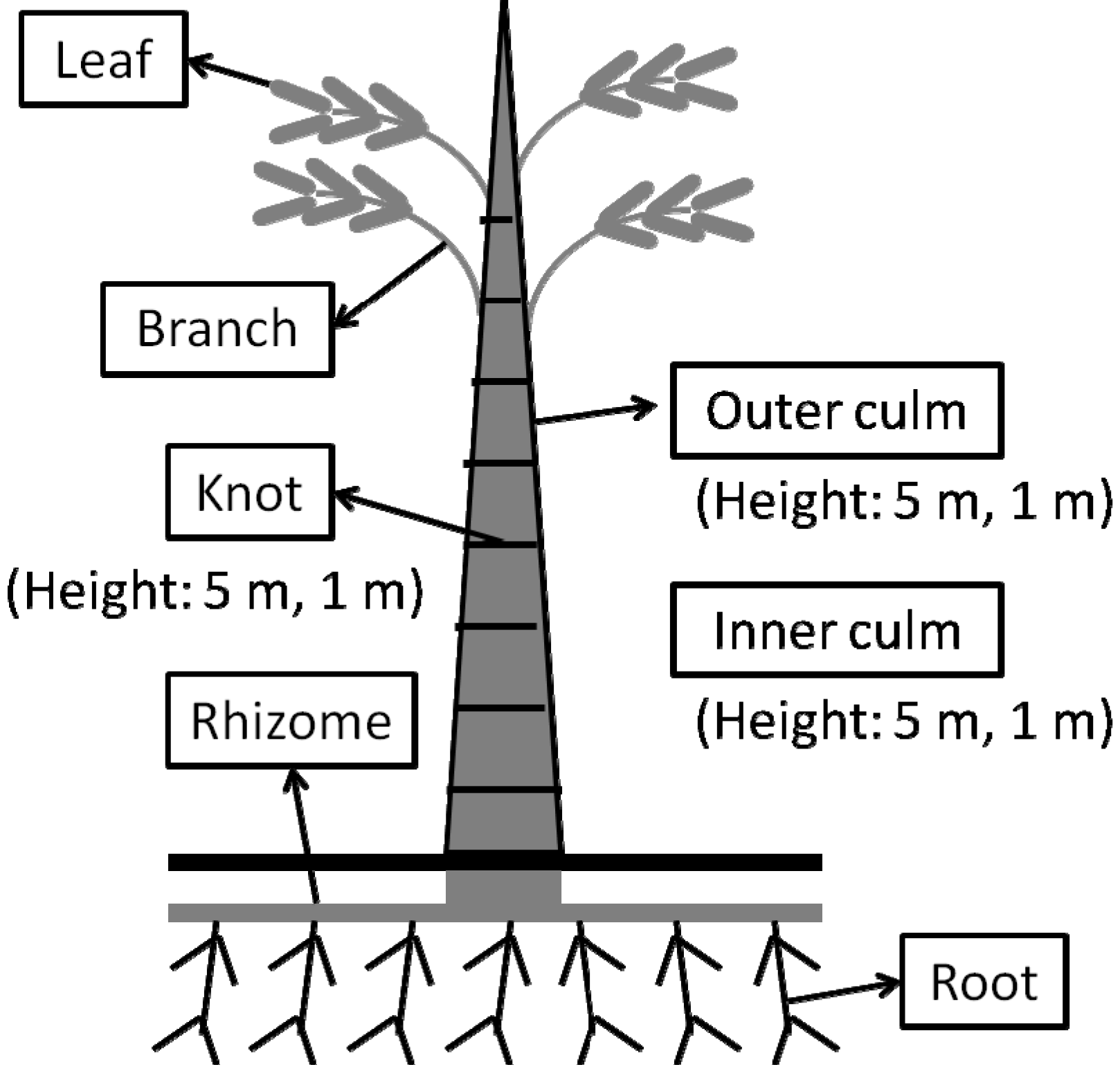
:1. Introduction
2. Results and Discussion
2.1. Activity on Melanin Biosynthesis
| Part | Ethanol extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 120 μg/mL | 60 μg/mL | 20 μg/mL | |||||||
| CV | MC | Type | CV | MC | Type | CV | MC | Type | |
| Leaf | 91.2 ± 1.06 | 105 ± 5.52 | - | 96.7 ± 8.10 | 99.0 ± 8.53 | - | 101 ± 1.98 | 103 ± 1.32 | - |
| Branch | 86.3 ± 1.71 | 56.0 ± 7.90 | A,C | 91.0 ± 2.74 | 75.6 ± 3.52 | - | 90.2 ± 0.84 | 84.6 ± 2.09 | - |
| Outer culm (5 m) | 86.9 ± 10.1 | 44.4 ± 5.64 | A,C | 98.9 ± 0.98 | 72.2 ± 1.55 | A | 112.9 ± 4.27 | 104 ± 2.34 | - |
| Outer culm (1 m) | 124 ± 9.08 | 49.5 ± 5.38 | A | 109 ± 1.20 | 79.8 ± 3.19 | A | 124 ± 9.08 | 112 ± 2.97 | - |
| Inner culm (5 m) | 98.8 ± 2.09 | 110 ± 2.67 | - | 88.5 ± 10.5 | 109 ± 9.68 | B,C | 98.0 ± 1.09 | 106 ± 8.01 | - |
| Inner culm (1 m) | 106 ± 1.80 | 142 ± 2.87 | B | 106 ± 5.63 | 151.9 ± 9.59 | B | 103 ± 3.32 | 134 ± 3.59 | B |
| Knot (5 m) | 93.6 ± 5.51 | 101 ± 9.90 | - | 90.5 ± 1.96 | 104 ± 0.69 | - | 93.4 ± 4.86 | 101 ± 3.27 | - |
| Knot (1 m) | 100 ± 6.22 | 133 ± 20.0 | B | 97.0 ± 7.14 | 119 ± 2.97 | B | 96.8 ± 6.46 | 107 ± 6.67 | - |
| Rhizome | 120 ± 2.97 | 137 ± 19.0 | - | 118 ± 1.88 | 144 ± 21.6 | B | 114 ± 4.44 | 121 ± 4.2 | - |
| Root | 91.5 ± 2.68 | 118 ± 6.78 | B | 88.6 ± 1.2 | 126 ± 5.53 | B, C | 104 ± 10.1 | 111 ± 12.7 | - |
| Part | Hot water extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 120 μg/mL | 60 μg/mL | 20 μg/mL | |||||||
| CV | MC | Type | CV | MC | Type | CV | MC | Type | |
| Leaf | 109 ± 9.06 | 114 ± 7.71 | - | 107 ± 8.35 | 108 ± 11.1 | - | 109 ± 9.06 | 143.8 ± 2.22 | B |
| Branch | 79.7 ± 10.7 | 84.8 ± 8.16 | C | 77.3 ± 3.67 | 87.2 ± 1.39 | C | 83.1 ± 7.42 | 109 ± 2.73 | B,C |
| Outer culm (5 m) | 121 ± 6.83 | 78.9 ± 6.10 | A | 125 ± 3.76 | 93.3 ± 13.0 | A | 138 ± 2.22 | 94.6 ± 6.76 | A |
| Outer culm (1 m) | 118 ± 2.81 | 125 ± 16.5 | - | 114 ± 2.99 | 104 ± 16.5 | - | 116 ± 6.16 | 117 ± 14.8 | - |
| Inner culm (5 m) | 97.5 ± 7.10 | 104 ± 6.62 | - | 114 ± 9.85 | 105 ± 15.2 | - | 96.2 ± 9.62 | 101 ± 2.82 | - |
| Inner culm (1 m) | 120 ± 5.10 | 113 ± 17.6 | - | 110 ± 15.0 | 108 ± 0.28 | - | 113 ± 0.76 | 102 ± 1.97 | - |
| Knot (5 m) | 79.1 ± 6.23 | 94.8 ± 5.69 | C | 85.8 ± 2.81 | 109 ± 9.61 | B,C | 77.7 ± 1.65 | 101 ± 1.86 | B,C |
| Knot (1 m) | 90.1 ± 12.9 | 99.5 ± 14.1 | - | 103 ± 17.5 | 103 ± 22.0 | - | 78.0 ± 8.30 | 96.8 ± 2.91 | C |
| Rhizome | 111 ± 3.12 | 88.9 ± 2.01 | A | 123 ± 9.64 | 97.3 ± 15.3 | A | 115 ± 7.03 | 93.2 ± 12.6 | A |
| Root | 99.9 ± 2.22 | 114 ± 10.4 | - | 94.3 ± 4.94 | 121 ± 9.18 | B | 98.2 ± 3.49 | 116 ± 11.2 | B |
| Part | Ethanol Extract | Hot Water Extract | ||||
|---|---|---|---|---|---|---|
| ORAC (mgTE/mg) | SOD Unit (U/μg) | ABTS IC50 (μg/mL) | ORAC (mgTE/mg) | SOD Unit (U/μg) | ABTS IC50 (μg/mL) | |
| Leaf | 0.07 ± 0.02 | nd | nd | 0.37 ± 0.08 | nd | 306.7 ± 5.7 |
| Branch | 0.69 ± 0.04 | 4.4 ± 1.0 | 350.6 ± 7.1 | 0.84 ± 0.01 | 0.6 ± 0.0 | 179.5 ± 3.6 |
| Outer culm (5 m) | 0.52 ± 0.07 | 0.2 ± 0.0 | nd | 0.65 ± 0.03 | 1.0 ± 0.3 | 113.7 ± 18.2 |
| Outer culm (1 m) | 0.18 ± 0.01 | 0.1 ± 0.0 | nd | 0.59 ± 0.05 | 0.8 ± 0.1 | 140.1 ± 1.4 |
| Inner culm (5 m) | 0.72 ± 0.09 | 0.9 ± 0.1 | 88.5 ± 0.8 | 0.29 ± 0.03 | nd | 198.3 ± 3.0 |
| Inner culm (1 m) | 1.35 ± 0.14 | 0.2 ± 0.0 | 373.8 ± 3.2 | 0.30 ± 0.00 | nd | 231.9 ± 4.9 |
| Knot (5 m) | 0.22 ± 0.00 | nd | nd | 0.29 ± 0.02 | nd | 245.0 ± 4.2 |
| Knot (1 m) | 0.22 ± 0.00 | nd | nd | 0.28 ± 0.01 | nd | 240.7 ± 1.9 |
| Rhizome | 0.71 ± 0.02 | 0.1 ± 0.0 | 171.5 ± 5.4 | 0.31 ± 0.00 | nd | 266.7 ± 6.8 |
| Root | 0.05 ± 0.03 | nd | nd | 0.54 ± 0.02 | 0.2 ± 0.0 | 209.7 ± 7.8 |
| Part | Ethanol Extract | Hot Water Extract | ||||
|---|---|---|---|---|---|---|
| Growth Inhibition | MIC/MBC (μg/mL) | Growth Inhibition | MIC/MBC (μg/mL) | |||
| Concentration (μg/mL) | Rate (% vs. Control) * | Concentration (μg/mL) | Rate (% vs. Control) * | |||
| Leaf | 600 | - | - | 600 | 98.1 ± 0.47 | 1200/1600 |
| Branch | 1200 | - | - | 1200 | 97.6 ± 1.61 | 1400/>1400 |
| Outer culm (5 m) | 600 | 97.8 ± 11.6 | 400/1600 | 600 | 13.7 ± 6.89 | nd |
| Outer culm (1 m) | 600 | 100 ± 0.47 | 400/1600 | 600 | 12.1 ± 9.30 | nd |
| Inner culm (5 m) | 600 | - | - | 600 | 99.5 ± 1.68 | >1600 |
| Inner culm (1 m) | 600 | - | - | 600 | - | - |
| Knot (5 m) | 600 | - | - | 600 | 31.2 ± 15.0 | nd |
| Knot (1 m) | 600 | - | - | 600 | - | - |
| Rhizome | 1200 | - | - | 1200 | 44.1 ± 12.9 | nd |
| Root | 1200 | - | - | 1200 | 52.4 ± 15.7 | nd |
| Part | IgE Production (%) | |
|---|---|---|
| Ethanol Extract | Hot Water Extract | |
| Leaf | 97.3 ± 38.9 | 57.2 ± 9.28 ** |
| Branch | 227 ± 95.8 | 103 ± 45.4 |
| Outer culm (5 m) | 144 ± 27.7 | 70.7 ± 13.1 * |
| Outer culm (1 m) | 137 ± 109 | 64.1 ± 6.47 ** |
| Inner culm (5 m) | 110 ± 39.6 | 64.1 ± 18.1 * |
| Inner culm (1 m) | 93.1 ± 15.0 | 66.9 ± 19.8 |
| Knot (5 m) | 115 ± 61.9 | 64.1 ± 10.3 ** |
| Knot (1 m) | 107 ± 39.8 | 73.8 ± 14.8 |
| Rhizome | 60.6 ± 29.8 | 62.8 ± 15.4 * |
| Root | 75.1 ± 31.0 | 73.7 ± 19.4 |
2.2. Antioxidant Activity
2.3. Antibacterial Activity
2.4. Anti-Allergy Activity
| Part | Comp. * | tR (min) | UV λ (nm) | MS | MS/MS | Tentative Identification | |
|---|---|---|---|---|---|---|---|
| [M+H]+ | Main Fragments | ||||||
| Leaf | 1 | 7.79 | 254,326 | 595.1222 | 563.2069 385.1727 401.2092 | 472.1109 325.0804 457.1063 379.0754 | Di-C,C-hexosyl apigenin |
| 2 | 9.75 | 254,326 | 583.2920 | 249.1044 331.6180 419.1789 532.1011 | - | Tricin derivative | |
| 3 | 10.99 | 254, 326 | 547.0304 | 214.9922 405.2008 316.5860 474.0304 | 391.0690 260.0637 419.1118 | Not identified | |
| 4 | 13.68 | 254,326 | 639.1865 | 561.3183 427.5450 357,1133 331.0914 | 331.0833 | O-hexosyl-O- deoxyhexosyl tricin | |
| 5 | 13.96 | 254,326 | 493.1227 | 235.0158 314.0666 | 331.0777 | O-hexosyl tricin | |
| Branch | 6 | 11.01 | 254,326 | 433.1361 | 313.0428 214.9618 | 283.0601 337.0809 415.0739 162.9025 | 6-C-glucosyl apigenin (isovitexin) |
| Outer culm (5 m) | 7 | 4.48 | 254 | 351.0937 | 196.9942 442.0793 253.0631 156.0012 | 269.3353 315.1813 211.5120 153.9859 | Not identified |
| 1 | 7.55 | 254,326 | 595.2048 | 401.1621 563.2534 385.1795 511.1788 | 383.1592 373.1058 318.5544 244.3389 | Di-C,C-hexosyl apigenin | |
| 4 | 13.65 | 254,326 | 639.1805 | 561.3528, 589.1020 315.0292 173.9611 | 331.0775 270.0903 415.4247 | O-hexosyl-O- deoxyhexosyl tricin | |
| Outer culm (1 m) | 1 | 7.70 | 254,326 | 595.1777 | 563.2181 385.2050 457.1007 214.9845 | 325.0885 427.1041 457.0921 379.0553 | Di-C,C-hexosyl apigenin |
| 9 | 12.34 | 254,326 | 549.1879 | 197.1128 384.5780 498.6190 | 447.1168 495.1613 | Not identified | |
| 4 | 13.57 | 254,326, | 639.2403 | 561.3504 215.0037 289.1380 401.0721 485.6067 | 331.0808 | O-hexosyl-O- deoxyhexosyl tricin | |
| Inner culm (5 m) | 1 | 7.71 | 254,326 | 595.1699 | 401.0721 215.0030 563.2233 379.1075 | 427.0928 457.1007 295.0754 379.0791 | Di-C,C-hexosyl apigenin |
| Inner culm (1 m) | 10 | 10.69 | 254 | 581.1780 | 401.1565 140.0316 214.9463 284.7471 | 305.0035 219.0867 131.0860 | Not identified |
| 11 | 10.92 | 254,326 | 581.2469, | 215.0359 256.0465 329.6107 155.9239 | 173.9575 | Not identified | |
| Knot (5 m) | 12 | 7.77 | 254,326 | 597.1854 | 214.9895 197.0173 256.0255 433.8491 | 149.0515 165.7342 223.7458 | Not identified |
| Knot (1 m) | 2 | 9.81 | 254,326 | 583.1954 | 249.1038 401.1687 331.5832 237.1147 | 131.0739 232.1788 231.0688 | Tricin derivative |
| 13 | 17.03 | 254,326 | 441.1956 | 354.2400 212.0524 154.9682 | 265.1592 177.0511 | Not identified | |
| Rhizome | 14 | 15.16 | 254,326 | 323.1311 | 256.0713 181.0395 196.9698 215.0161 240.9718 | 169.2761 | Not identified |
| 15 | 15.92 | 254,326 | 353.1676 | 181.0075 156.0012 255.9951 214.9742 | 177.0655 145.0326 337.1738 | Not identified | |
| 16 | 16.52 | 254,326 | 411.1811 | 215.0015 206.1046 266.0636 367.1154 | 235.1485 147.0246 265.1572 177.0882 | Not identified | |
| 13 | 17.01 | 254, 326 | 441.2149 | 289.0373 197.0155 154.0150 255.9951 | 265.1526 177.0535 145.0496 | Not identified | |
| 17 | 18.43 | 254,326 | 455.2147 | 181.0407 214.9568 197.0268 381.6146 266.0714 308.6563 | 173.9925 124.2863 249.5310 | Not identified | |
| 18 | 22.73 | 254,326 | 445.1614 | 214.9897 181.0442 197.0006 317.1962 405.2211 283.1105 | 427.1142 177.9142 362.6228 114.6710 | Not identified | |
| Root | 1 | 7.70 | 254,326 | 595.1482 | 196.9985 498.0875 542.1194 325.0225 249.1168 | 409.1006 457.1415 369.0885 421.0878 439.1011 | Di-C,C-hexosyl apigenin |
| 2 | 9.90 | 254,326 | 583.2460 | 249.1353 331.5924 605.1899 360.0969 214.9603 281.0794 403.5934 | 207.3230 520.5871 286.0984 412.9828 388.1342 | Not identified | |
| 19 | 11.05 | 245,326 | 579.1288 | 214.9826 247.0254 350.0942 164.0768 | 411.1128 429.1186 393.1004 349.0813 409.1005 295.0939 | Not identified | |
| 20 | 12.37 | 245,326 | 549.1083 | 531.1381 197.0006 457.1618 337.1038 382.8247 139.9865 | 531.1381 197.0006 457.1618 337.1038 382.8247 139.9865 | Di-C-glycosyl apigenin | |
| 5 | 13.92 | 245,326 | 493.1157 | 295.0853 338.5214 197.0107 475.3166 | 331.0791 442.3596 244.4431 | O-hexosyl tricin | |
| Part | Comp. * | tR (min) | UV λ (nm) | MS | MS/MS | Tentative Identification | |
|---|---|---|---|---|---|---|---|
| [M+H]+ | Main Fragments | ||||||
| Leaf | 21 | 8.36 | 254,278 | 346.0437 | 206.9890 235.0134 173.0079 242.0372 | 145.0514 292.1701 177.0293 313.7123 | Not identified |
| 22 | 12.69 | 254,278 | 355.0828 | 207.0098 146.9837 275.0457 235.0089 185.1625 | 174.9783 163.2186 | Chlorogenic acid | |
| 23 | 19.19 | 254,278 | 449.1190 | 207.0091 234.9587 243.0011 177.0516 285.0846 377.4037 | 299.0534 353.0652 383.0690 339.0555 395.0833 | 8-C-glucosyl luteolin (orientin) | |
| 24 | 19.79 | 254,278 | 449.0907 | 431.0489 301.1575 206.9786 215.0161 | 299.0532, 353.0659, 395.0849, 463.4953 329.0699, 383.0960 | 6-C-glucosyl luteolin (isoorientin) | |
| 25 | 20.86 | 254,278 | 433.1147 | 206.9915 234.0091 174.9798 251.1581 279.0238 | 177.7364 245.2327 100.8017 | 8-C-glucosyl apigenin (vitexin) | |
| 6 | 22.32 | 254,278 | 433.1255 | 175.0077 313.0549 455.0990 | 168.5285 | 6-C-glucosyl apigenin (isoviterxin) | |
| 5 | 25.95 | 254,278 | 493.1481 | 206.9978 371.0754 159.0127 351.1284 | 331.0757 | O-hexosyl tricin | |
| 4 | 26.75 | 254,278 | 639.1185 | 191.0127 207.0049 235.0432 253.1692 460.8544 | 331.0811 315.0479 | O-hexosyl-O- deoxyhexosyl tricin | |
| Branch | 21 | 8.57 | 254,278 | 346.0656 | 206.9941 234.9992 174.9993 191.0522 | 248.2391 | Not identified |
| 1 | 16.93 | 254, 278 | 595.1387 | 579.1455 371.1160 249.1300 311.0476 235.0080 207.0031 | 457.1221 325.0685 427.1048 379.0890 295.0745 | Di-C,C-hexosyl apigenin | |
| Outer culm (5 m) | 21 | 8.65 | 254, 278 | 346.0495 | 206.9909 235.0080 158.9887 174.9798 193.0579 | 152.0649 257.2977 172.7825 | Not identified |
| 1 | 16.90 | 254, 278 | 595.1733 | 579.2583 457.1415 371.0852 249.1032 311.0364 206.9530 | 379.0866 457.1184 427.1094 325.0682 | Di-C,C-hexosyl apigenin | |
| 4 | 26.62 | 254, 278 | 639.1777 | 557.1886 441.1388 355.1725 175.0428 159.0067 | 331.0788 315.0678 270.0594 285.0364 | O-hexosyl-O-deoxyhexosyl tricin | |
| Outer culm (1 m) | 21 | 8.59 | 254, 278 | 346.0550 | 207.0078 174.9546 235.0164 218.0567 | 152.0448 202.7962 | Not identified |
| 1 | 16.89 | 254, 278 | 595.1143 | 579.1475 249.1167 371.1295 207.0176 175.0050 | 427.0888 379.0915 295.0598 | Di-C,C-hexosyl apigenin | |
| 26 | 25.86 | 254, 278 | 295.0849 | 207.0089 219.0139 174.9589 147.0010 | 135.8380 178.2954 | Not identified | |
| Inner culm (5 m) | 27 | 16.95 | 254, 278 | 165.0803 | 146.9779 | - | p-courmaric acid |
| Inner culm (1 m) | 27 | 16.88 | 254, 278 | 165.0865 | 146.9779 | - | p-courmaric acid |
| Knot (5 m) | 27 | 17.06 | 254, 278 | 165.0806 | 146.9779 | - | p-courmaric acid |
| Knot (1 m) | 27 | 16.89 | 254, 278 | 165.2503 | 147.0356 | - | p-courmaric acid |
| Rhizome | 28 | 7.05 | 254,278 | 330.0130 | 206.9530 234.9505 174.9735 266.0403 | 221.4443 259.8027 104.8472 | Not identified |
| 21 | 8.66 | 254,278 | 346.0354 | 206.9626 233.9144 174.9779 214.9463 | 152.0740 174.0595 | Not identified | |
| 27 | 16.92 | 254,278 | 165.3803 | 146.9779 159.0007 | 132.8580 | p-courmaric acid | |
| Root | 27 | 16.91 | 254,278 | 165.0853 | 146.9894 159.0127 | - | p-courmaric acid |
2.5. Phytochemical Profile
3. Experimental
3.1. Plant Materials
3.2. Melanin Biosynthesis Assay
3.2.1. Cell Viability
3.2.2. Determination of Melanin Content
3.3. Antioxidant Assays
3.3.1. Oxygen Radical Absorbance Capacity Assay
3.3.2. Superoxide Dismutase-Like Activity
3.3.3 ABTS Radical Cation Decolorization Assay
3.4. Antibacterial Assay
3.5. Immunoglobulin E (IgE) Production Assay
3.6. LCMS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, H.-O.; Baek, S.-H.; Han, D.-M. Antimicrobial effects of Chamaecyparis obtusa essential oil. Korean J. Appl. Microbiol. Biotechnol. 2001, 29, 253–257. [Google Scholar]
- Zhang, Y.; Jiao, J.; Liu, C.; Wu, X.; Zhang, Y. Isolation and purification of four flavone C-glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. Food Chem. 2008, 107, 1326–1336. [Google Scholar]
- Lu, B.-Y.; Zhang, Y.; Wu, X.-Q. Advances in studies on antioxidative activity and cardio-cerebrovascular pharmacology of bamboo-leaf-flavonoids. Linchan Huaxue Yu Gongye 2005, 25, 120–124. [Google Scholar]
- Jiao, J.; Zhang, Y.; Liu, C.; Liu, J.; Wu, X. Separation and purification of tricin from an antioxidant product derived from bamboo leaves. J. Agric. Food Chem. 2007, 55, 10086–10092. [Google Scholar] [CrossRef]
- Hasegawa, T.; Tanaka, A.; Hosoda, A.; Takano, F.; Ohta, T. Antioxidant C-glycosyl flavones from the leaves of Sasa kurilensis var. gigantea. Phytochemistry 2008, 69, 1419–1424. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.; Bao, B. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 2006, 20, 872–876. [Google Scholar] [CrossRef]
- Kurokawa, T.; Itagaki, S.; Yamaji, T.; Nakata, C.; Noda, T.; Hirano, T.; Iseki, K. Antioxidant activity of a novel extract from bamboo grass (AHSS) against ischemia-reperfusion injury in rat small intestine. Biol. Pharm. Bull. 2006, 29, 2301–2303. [Google Scholar] [CrossRef]
- Nishina, A.; Hasegawa, K.I.; Uchibori, T.; Seino, H.; Osawa, T. 2,6-dimethoxy-p-benzoquinone as an antibacterial substance in the culm of Phyllostachys heterocycla var. Pubescens, a species of thick-stemmed bamboo. J. Agric. Food Chem. 1991, 39, 266–269. [Google Scholar] [CrossRef]
- Fujimura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Amino acid sequence and antimicrobial activity of chitin-binding peptides, Pp-AMP 1 and Pp-AMP 2, from Japanese bamboo shoots (Phyllostachys pubescens). Biosci. Biotechnol. Biochem. 2005, 69, 642–645. [Google Scholar] [CrossRef]
- Tanaka, A.; Shimizu, K.; Kondo, R. Antibacterial compounds from shoot skins of moso bamboo (Phyllostachys pubescens). J. Wood Sci. 2013, 59, 155–159. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Sakai, K.; Achiwa, Y.; Imai, K.; Komiya, T. Isolation of antioxidative compounds from bamboo shoots sheath. Nippon Shokuhin Kagaku Kogaku Kaishi 1999, 46, 491–493. [Google Scholar] [CrossRef]
- Fujita, S. Ecological and mechanical aspect of bamboo. Biol. Resour. 2012, 6, 2–11. (In Japanese) [Google Scholar]
- Guo, X.F.; Yue, Y.D.; Tang, F.; Wang, J.; Yao, X. Antioxidant properties of major flavonoids and subfractions of the extract of Phyllostachys pubescens leaves. J. Food Biochem. 2012, 37, 501–509. [Google Scholar]
- Zhang, Z.; Wang, X.; Yu, S.; Zhao, M. Isolation and antioxidant activities of polysaccharides extracted from the shoots of Phyllostachys edulis (Carr.). Int J. Biol Macromol 2011, 49, 454–457. [Google Scholar] [CrossRef]
- Higa, J.K.; Panee, J. Bamboo extract reduces interleukin 6 (IL-6) overproduction under lipotoxic conditions through inhibiting the activation of NF-kappaB and AP-1 pathways. Cytokine 2011, 55, 18–23. [Google Scholar] [CrossRef]
- Lin, Y.; Collier, A.C.; Liu, W.; Berry, M.J.; Panee, J. The inhibitory effect of bamboo extract on the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer. Phytother Res. 2008, 22, 1440–1445. [Google Scholar] [CrossRef]
- Tanaka, A.; Kim, H.J.; Oda, S.; Shimizu, K.; Kondo, R. Antibacterial activity of moso bamboo shoot skin (Phyllostachys pubescens) against Staphylococcus aureus. J. Wood Sci. 2011, 57, 542–544. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentao, P.; Tomas-Barberan, F.A. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar]
- Meng, T.X.; Furuta, S.; Fukamizu, S.; Yamamoto, R.; Ishikawa, H.; Arung, E.T.; Shimizu, K.; Kondo, R. Evaluation of biological activities of extracts from the fruiting body of Pleurotus citrinopileatus for skin cosmetics. J. Wood Sci. 2011, 57, 452–458. [Google Scholar] [CrossRef]
- Breuer, K.; HÄussler, S.; Kapp, A.; Werfel, T. Staphylococcus aureus: Colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br. J. Dermatol. 2002, 147, 55–61. [Google Scholar] [CrossRef]
- Iwamoto, A.; Mitsuda, K.; Inoue, A.; Kato, T.; Inoue, Y.; Kawahara, H. Purification and identification of an IgE suppressor from strawberry in an in vitro immunization system. Cytotechnology 2012, 64, 309–314. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; de Beer, T.; Deforce, D.; Heyerick, A. Phenolic compounds and anti-oxidant capacity of twelve morphologically heterogeneous bamboo species. Phytochem. Anal. 2012, 23, 433–443. [Google Scholar] [CrossRef]
- Lv, Z.; Dong, J.; Zhang, B. Rapid identification and detection of flavonoids compounds from bamboo leaves by LC-(ESI)-IT-TOF/MS. BioResources 2012, 7, 1405–1418. [Google Scholar]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Lagana, A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Huang, C.S.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Wang, T.S.; Chen, H.W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Czyz, J.; Madeja, Z.; Irmer, U.; Korohoda, W.; Hulser, D.F. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int J. Cancer 2005, 114, 12–18. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Iwaoka, E.; Oku, H.; Iinuma, M.; Ishiguro, K. Allergy-preventive effects of the flowers of Impatiens textori. Biol. Pharm. Bull. 2010, 33, 714–716. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Varvara, G.; Murmura, G.; Caraffa, A.; Antinolfi, P.; Toniato, E.; Pantalone, A.; Neri, G.; Frydas, S.; et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 955–959. [Google Scholar]
- Li, S.Y.; Chang, C.Q. Biological effects of chlorogenic acid and body health. Wei Sheng Yan Jiu 2005, 34, 762–764. [Google Scholar]
- Oku, H.; Ogawa, Y.; Iwaoka, E.; Ishiguro, K. Allergy-preventive effects of chlorogenic acid and iridoid derivatives from flower buds of Lonicera japonica. Biol. Pharm. Bull. 2011, 34, 1330–1333. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Beeson, T.D.; Mueller, P.J.; Hsiang, J.; Lewis, K. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem. Syst. Ecol. 2001, 29, 793–798. [Google Scholar]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Romanova, D.; Vachalkova, A.; Cipak, L.; Ovesna, Z.; Rauko, P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma 2001, 48, 104–107. [Google Scholar]
- Arung, E.T.; Shimizu, K.; Kondo, R. Inhibitory effect of artocarpanone from Artocarpus heterophyllus on melanin biosynthesis. Biol. Pharm. Bull. 2006, 29, 1966–1969. [Google Scholar]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogasawara, Y.; Endou, K.; Fujimori, S.; Koyama, M.; Akano, H. Phenolic compounds responsible for the superoxide dismutase-like activity in high-Brix apple vinegar. J. Agric. Food Chem. 2010, 58, 10124–10132. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, A.; Zhu, Q.; Tan, H.; Horiba, H.; Ohnuki, K.; Mori, Y.; Yamauchi, R.; Ishikawa, H.; Iwamoto, A.; Kawahara, H.; et al. Biological Activities and Phytochemical Profiles of Extracts from Different Parts of Bamboo (Phyllostachys pubescens). Molecules 2014, 19, 8238-8260. https://doi.org/10.3390/molecules19068238
Tanaka A, Zhu Q, Tan H, Horiba H, Ohnuki K, Mori Y, Yamauchi R, Ishikawa H, Iwamoto A, Kawahara H, et al. Biological Activities and Phytochemical Profiles of Extracts from Different Parts of Bamboo (Phyllostachys pubescens). Molecules. 2014; 19(6):8238-8260. https://doi.org/10.3390/molecules19068238
Chicago/Turabian StyleTanaka, Akinobu, Qinchang Zhu, Hui Tan, Hiroki Horiba, Koichiro Ohnuki, Yasuhiro Mori, Ryoko Yamauchi, Hiroya Ishikawa, Akira Iwamoto, Hiroharu Kawahara, and et al. 2014. "Biological Activities and Phytochemical Profiles of Extracts from Different Parts of Bamboo (Phyllostachys pubescens)" Molecules 19, no. 6: 8238-8260. https://doi.org/10.3390/molecules19068238





