Trithiocyanurate Complexes of Iron, Manganese and Nickel and Their Anticholinesterase Activity
Abstract
:1. Introduction
2. Results and Discussion
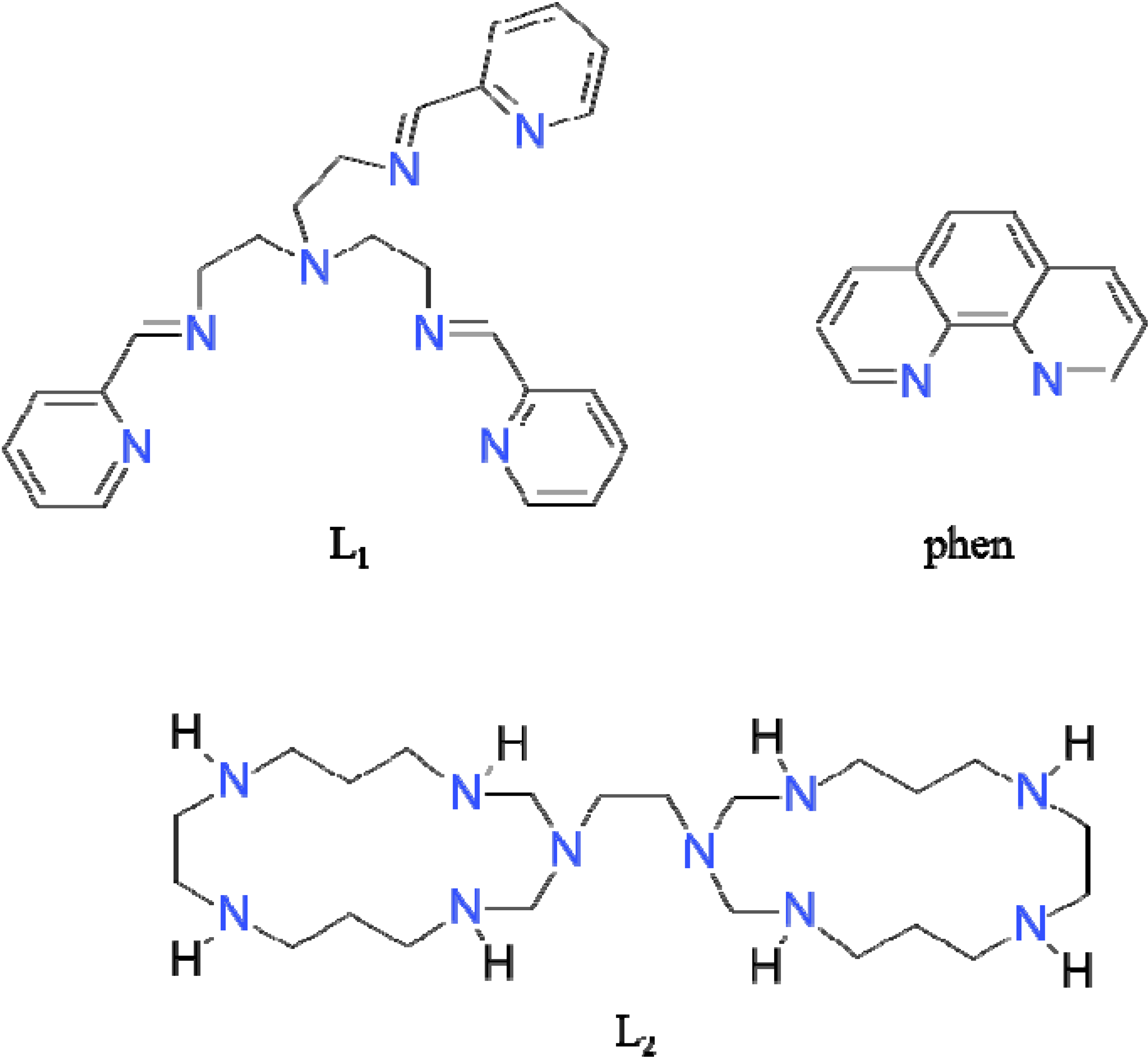
2.1. Synthesis and Spectral Study
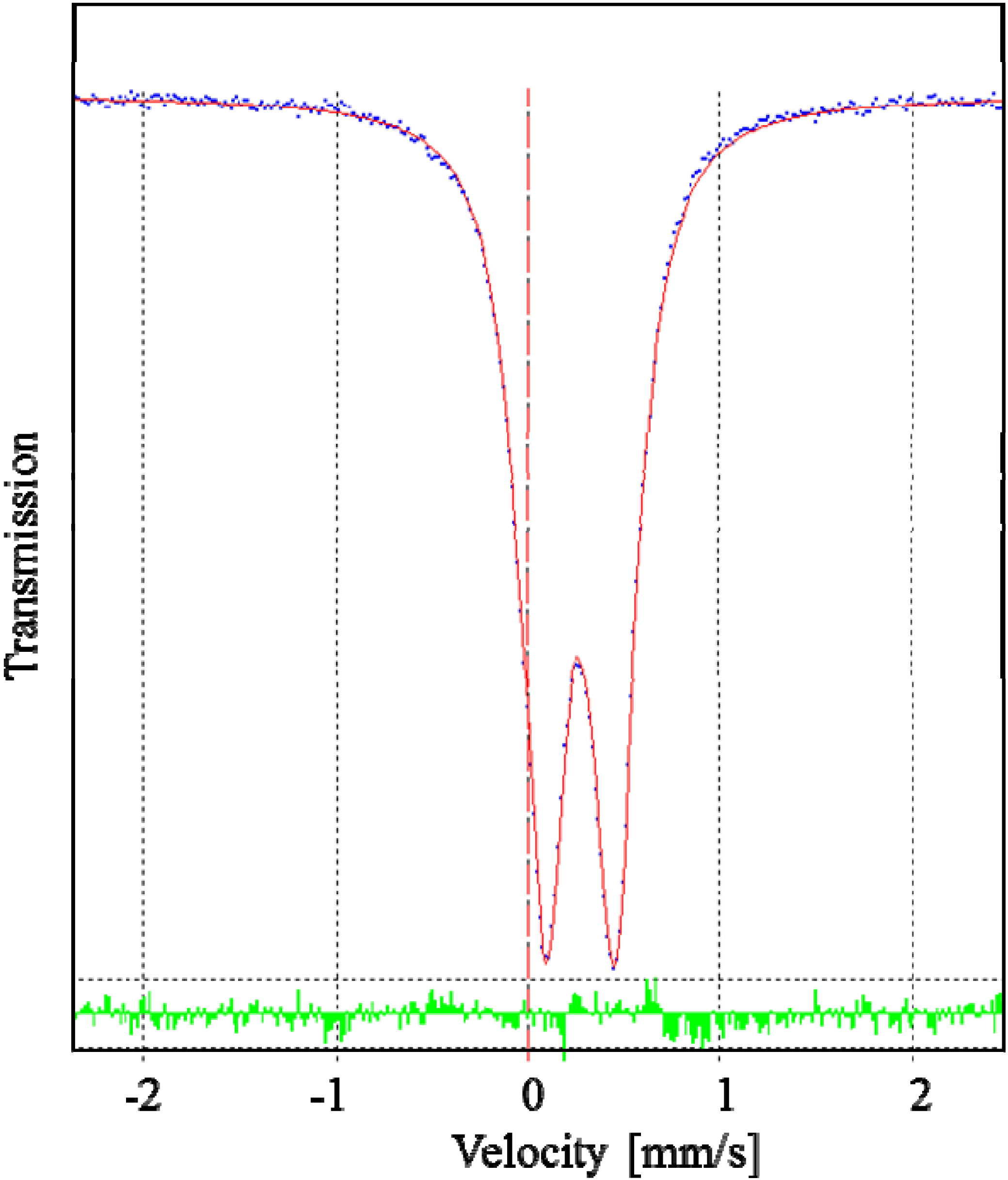
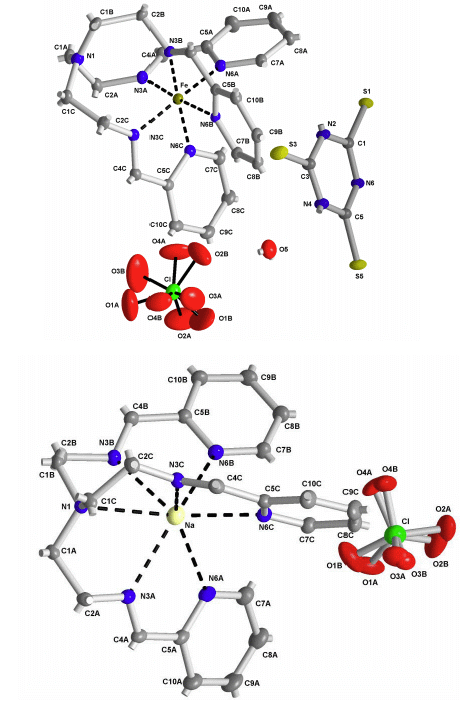
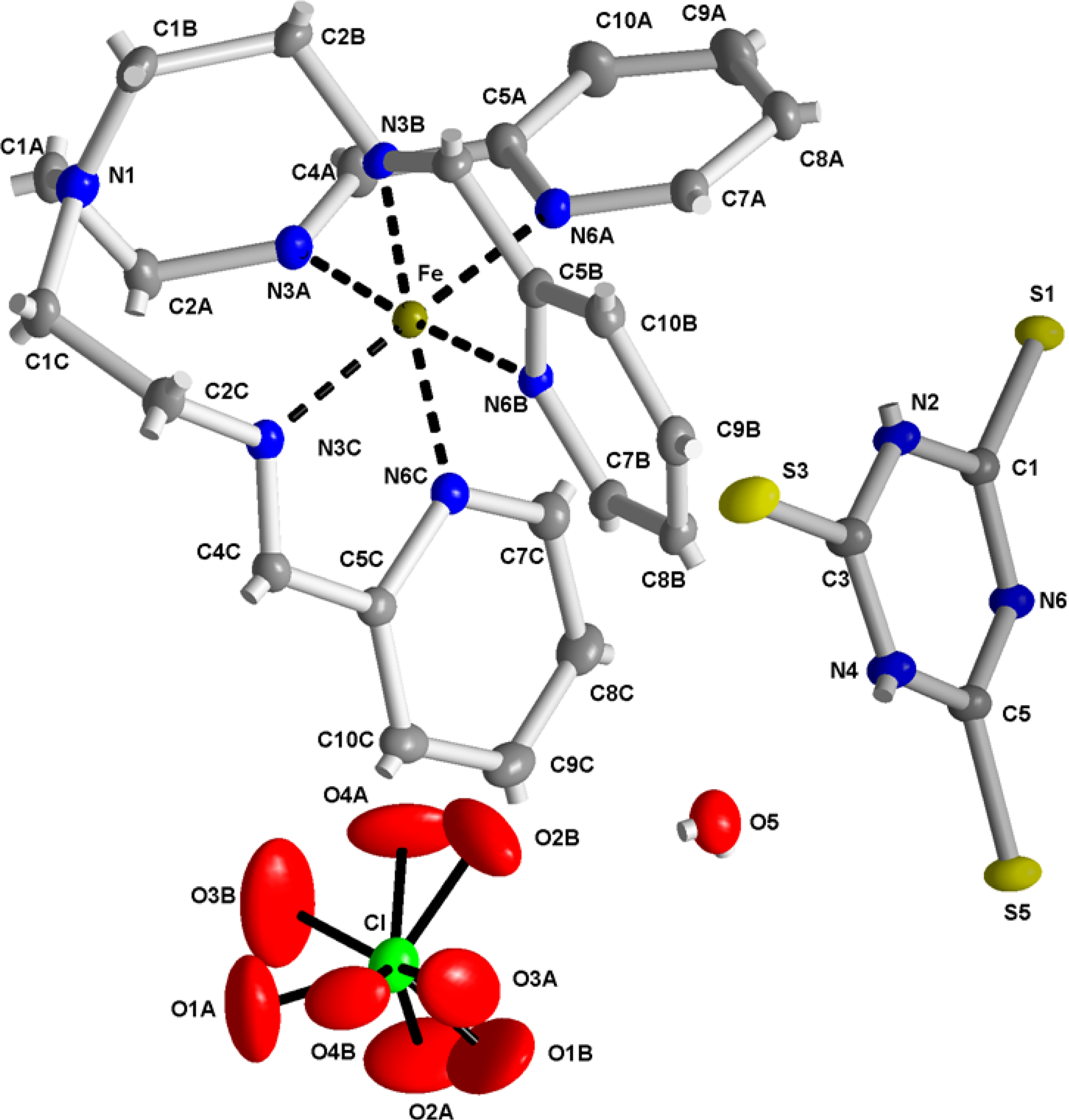
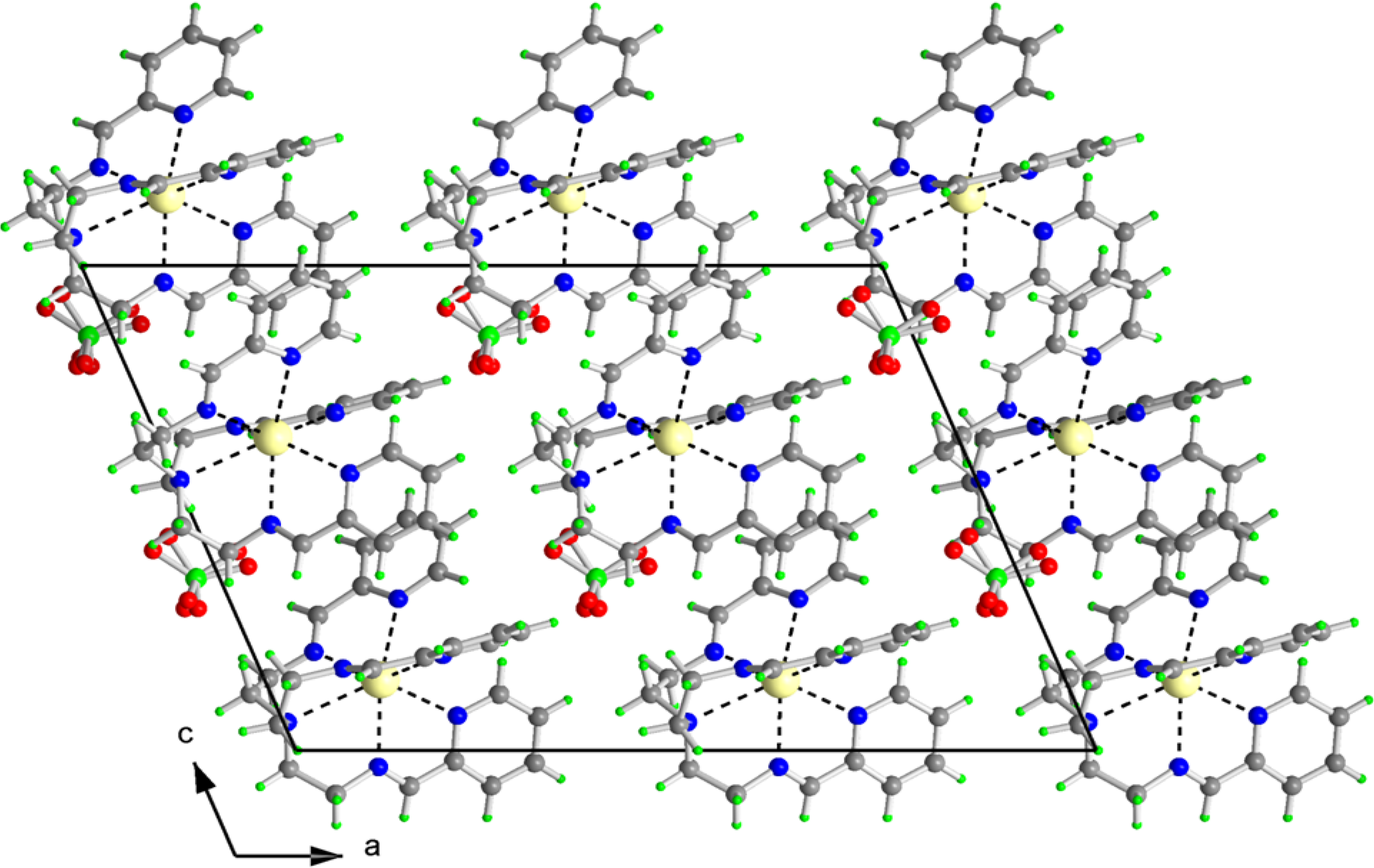
2.2. X-ray Structures of [Fe(L1)](ttcH2)(ClO4)·EtOH·H2O (1) and Na(L1)ClO4 (4)
| Fe-N3B | 1.9527(16) | N3B-Fe-N3C | 96.12(7) | N3C-Fe-N6A | 174.37(7) |
| Fe-N3A | 1.9569(16) | N3A-Fe-N3C | 96.15(7) | N6C-Fe-N6A | 94.01(7) |
| Fe-N3C | 1.9635(16) | N3B-Fe-N6C | 171.87(7) | N3B-Fe-N6B | 80.89(6) |
| Fe-N6C | 1.9720(16) | N3A-Fe-N6C | 91.45(7) | N3A-Fe-N6B | 175.87(7) |
| Fe-N6A | 1.9764(16) | N3C-Fe-N6C | 81.10(7) | N3C-Fe-N6B | 87.31(6) |
| Fe-N6B | 1.9997(15) | N3B-Fe-N6A | 89.10(7) | N6C-Fe-N6B | 91.32(6) |
| N3B-Fe-N3A | 96.45(7) | N3A-Fe-N6A | 81.11(7) | N6A-Fe-N6B | 95.64(6) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|
| O5-H52...O2B | 0.931(18) | 1.96(2) | 2.825(7) | 153 |
| O5-H52...O4A | 0.931(18) | 2.35(2) | 3.243(10) | 160 |
| O5-H52...O1B | 0.931(18) | 2.54(3) | 3.334(9) | 144 |
| O5-H52...Cl | 0.931(18) | 2.84(2) | 3.765(2) | 171 |
| N2-H2...S5i | 0.88 | 2.52 | 3.3879(17) | 169 |
| N4-H4...S1ii | 0.88 | 2.40 | 3.2418(17) | 160 |


| Na-N3A | 2.5280(14) | N3A-Na-N6C | 115.01(5) | N6C-Na-N6A | 88.66(4) |
| Na-N3C | 2.5338(13) | N3C-Na-N6C | 64.88(4) | N6B-Na-N6A | 90.08(4) |
| Na-N3B | 2.5406(13) | N3B-Na-N6C | 141.07(5) | N3A-Na-N1 | 65.01(4) |
| Na-N6C | 2.6224(13) | N3A-Na-N6B | 145.06(5) | N3C-Na-N1 | 64.31(4) |
| Na-N6B | 2.6300(14) | N3C-Na-N6B | 110.99(5) | N3B-Na-N1 | 65.04(4) |
| Na-N6A | 2.7353(14) | N3B-Na-N6B | 65.02(4) | N6C-Na-N1 | 127.09(4) |
| Na-N1 | 2.8431(12) | N6C-Na-N6B | 84.78(4) | N6B-Na-N1 | 126.93(4) |
| N3A-Na-N3C | 103.63(4) | N3A-Na-N6A | 63.49(4) | N6A-Na-N1 | 126.25(4) |
| N3A-Na-N3B | 103.60(4) | N3C-Na-N6A | 143.27(5) | N6C-Na-N6A | 88.66(4) |
| N3C-Na-N3B | 102.28(4) | N3B-Na-N6A | 114.01(4) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|
| C2B-H2B1...O3Bi | 0.99 | 2.56 | 3.483(11) | 154 |
| C7A-H7A...O1B | 0.95 | 2.59 | 3.264(10) | 128 |
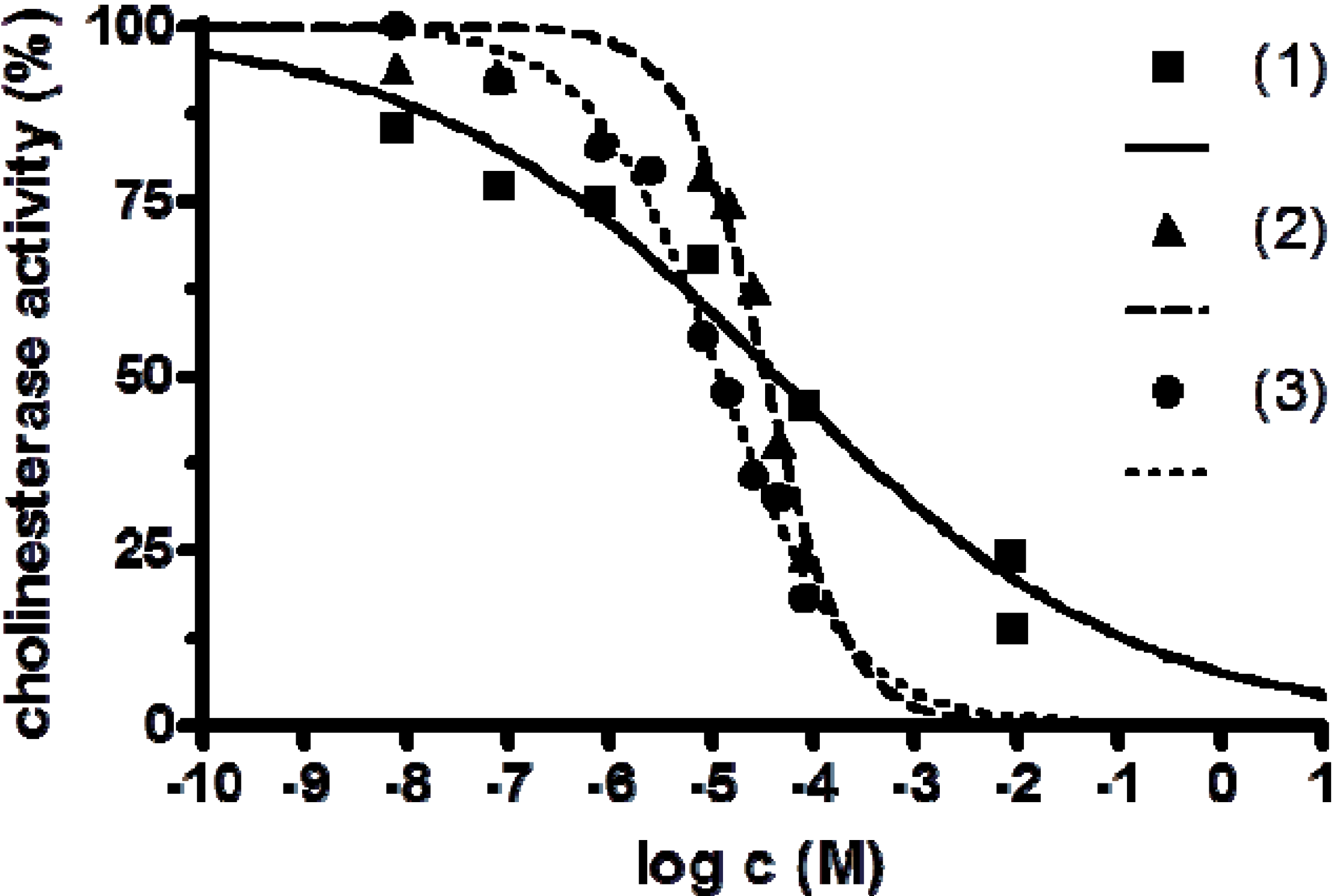
2.3. Anticholinesterase Activity
| Compound | IC50 [M] | 95% CI [M] | HillSlope |
|---|---|---|---|
| [Fe(L1)](ttcH2)(ClO4)·EtOH·H2O ( 1) | 4.35 × 10−5 | 1.34 × 10−5–1.41 × 10−4 | 0.0285 |
| [Mn3(phen)6(ttc)](ClO4)3 ( 2) | 3.34 × 10−5 | 2.28 × 10−5–4.88 × 10−5 | 0.2280 |
| Ni2(L2)(ttcH)(ClO4)2·6H2O·EtOH ( 3) | 1.20 × 10−5 | 9.46 × 10−6–1.51 × 10−5 | 0.0552 |
| FeSO4 | >10−3 | - | - |
| MnSO4 | >10−3 | - | - |
| NiSO4 | 1.29 × 10−2 | −0.3920 |
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of the Complexes
3.2.1. [Fe(L1)](ttcH2)(ClO4)·EtOH·H2O (1)
3.2.2. [Mn3(phen)6(ttc)](ClO4)3 (2)
3.2.3. Ni2(L2)(ttcH)(ClO4)2·6H2O·EtOH (3)
3.2.4. Na(L1)ClO4 (4)
3.3. X-ray Crystallography
3.4. Biological Activity Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bailey, J.R.; Hatfield, M.J.; Henke, K.R.; Krepps, M.K.; Morris, J.L.; Otieno, T.; Simonetti, K.D.; Wall, E.A.; Atwood, D.A. Transition metal complexes of 2,4,6-trimercapto-1,3,5-triazine (TMT): Potential precursors to nanoparticulate metal sulfides. J. Organomet. Chem. 2001, 623, 185–190. [Google Scholar] [CrossRef]
- Henke, K.R.; Robertson, D.; Krepps, M.K.; Atwood, D.A. Chemistry and stability of precipitates from aqueous solutions of 2,4,6-trimercaptotriazine, trisodium salt, nonahydrate (TMT-55) and mercury (II) chloride. Water Res. 2000, 34, 3005–3013. [Google Scholar] [CrossRef]
- Matlock, M.M.; Henke, K.R.; Atwood, D.A. Effectiveness of commercial reagents for heavy metal removal from water with new insights for future chelate designs. J. Hazard. Mater. 2002, 92, 129–142. [Google Scholar] [CrossRef]
- Liao, D.M.; Luo, Y.B.; Yu, P.; Chen, Z.G. Chemistry of copper trimercaptotriazine (TMT) compounds and removal of copper from copper-ammine species by TMT. Appl. Organomet. Chem. 2006, 20, 246–253. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Rosso, V.W.; Lust, D.A.; Bernot, P.J.; Grosso, J.A.; Modi, S.P.; Rusowicz, A.; Sedergran, T.C.; Simpson, J.H.; Srivastava, S.K.; Humora, M.J.; et al. Removal of palladium from organic reaction mixtures by trimercaptotriazine. Org. Process Res. Dev. 1997, 1, 311–314. [Google Scholar] [CrossRef]
- Iltzsch, M.H.; Klenk, E.E. Structure-activity relationship of nucleobase ligands of uridine phosphorylase from toxoplasma-gondii. Biochem. Pharmacol. 1993, 46, 1849–1858. [Google Scholar] [CrossRef]
- Iltzsch, M.H.; Tankersley, K.O. Structure-activity relationship of ligands of uracil phosphoribosyltransferase from toxoplasma-gondii. Biochem. Pharmacol. 1994, 48, 781–791. [Google Scholar] [CrossRef]
- Javaid, Z.Z.; el Kouni, M.H.; Iltzsch, M.H. Pyrimidine nucleobase ligands of orotate phosphoribosyltransferase from Toxoplasma gondii. Biochem. Pharmacol. 1999, 58, 1457–1466. [Google Scholar] [CrossRef]
- Kar, S.; Miller, T.A.; Chakraborty, S.; Sarkar, B.; Pradhan, B.; Sinha, R.K.; Kundu, T.; Ward, M.D.; Lahiri, G.K. Synthesis, mixed valence aspects and non-linear optical properties of the triruthenium complexes {(bpy)(2)Ru-II}(3)(L) (3+) and {(phen)(2)Ru-II}(3)(L) (3+) (bpy=2,2 '-bipyridine, phen=1,10-phenanthroline and L3-=1,3,5-triazine-2,4,6-trithiol). Dalton Trans. 2003, 2003, 2591–2596. [Google Scholar]
- Kar, S.; Pradhan, B.; Sinha, K.; Kundu, T.; Kodgire, P.; Rao, K.K.; Puranik, V.G.; Lahiri, G.K. Synthesis, structure, redox, NLO and DNA interaction aspects of {(L'(-)''')(2)Ru-II}(3)(mu(3)-L) (3+) and (L')(2)Ru-II(NC5H4S-) (+) L3-=1,3,5-triazine-2,4,6-trithiolato, L'(-)''' = arylazopyrid. Dalton Trans. 2004, 2004, 1752–1760. [Google Scholar]
- Kopel, P.; Dolezal, K.; Machala, L.; Langer, V. Synthesis, characterization and screening of biological activity of Zn(II), Fe(II) and Mn(II) complexes with trithiocyanuric acid. Polyhedron 2007, 26, 1583–1589. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Zboril, R.; Marek, J. Synthesis, X-ray and Mossbauer study of iron(II) complexes with trithiocyanuric acid (ttcH(3)). The X-ray structures of Fe(bpy)(3) (ttcH) center dot 2bpy center dot 7H(2)O and Fe(phen)(3) (ttcH(2))(ClO4) center dot 2CH(3)OH center dot 2H(2)O. Polyhedron 2004, 23, 2193–2202. [Google Scholar]
- Kopel, P.; Travnicek, Z.; Panchartkova, R.; Biler, M.; Marek, J. Coordination compounds of nickel with trithiocyanuric acid. Part II. Crystal and molecular structure of Ni(taa)(ttcH) (taa = tris-(2-aminoethyl)amine, ttcH(3) = trithiocyanuric acid). Transit. Met. Chem. 1999, 24, 239–243. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Kvitek, L.; Biler, M.; Pavlicek, M.; Sindelar, Z.; Marek, J. Coordination compounds of nickel with trithiocyanuric acid. Part IV. Structure of Ni(pmdien)(ttcH) (pmdien = N, N, N', N', N''-pentamethyldiethylenetriamine, ttcH(3) = trithiocyanuric acid). Transit. Met. Chem. 2001, 26, 282–286. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Kvitek, L.; Cernosek, Z.; Wrzeszcz, G.; Marek, J. Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes of trithiocyanuric acid: The structure of {N, N'-bis(3-aminopropyl)-1,3-propane-diamine}-(trithiocyanurato)nickel(II). J. Coord. Chem. 2003, 56, 1–11. [Google Scholar] [CrossRef]
- Marek, J.; Kopel, P.; Travnicek, Z. N, N'-bis(3-aminopropyl)ethylenediamine-kappa N-4, N', N'', N''' (trithio-cyanurato-kappa N-2,S)zinc(II) ethanol solvate. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 2003, 59, M558–M560. [Google Scholar] [CrossRef]
- Ienco, A.; Midollini, S.; Orlandini, A.; Vacca, A. Complexes formed from 2,4,6-trimercaptotriazine (H3TMT): Synthesis and structural characterization of M(PhP(CH2CH2P(Ph)(2))(2)(HTMT), M = Ni(II), Pd(II), Pt(II). Inorg. Chim. Acta 2004, 357, 2615–2623. [Google Scholar] [CrossRef]
- Yamanari, K.; Kushi, Y.; Yamamoto, M.; Fuyuhiro, A.; Kaizaki, S.; Kawamoto, T. Photochemical synthesis and crystal structures of tetrakis(ethane-1,2-diamine)dicobalt(III) complexes bridged by 1,3,5-triazine-2,4,6-trithionata and 4-oxo-1,3,5-triazine-2,6-dithionate. J. Chem. Soc.-Dalton Trans. 1993, 1993, 3715–3721. [Google Scholar]
- Cecconi, F.; Ghilardi, C.A.; Midollini, S.; Orlandini, A. Organomercury derivatives of the 2,4,6-trimercaptotriazine (H3TMT). X-ray crystal structure of (HgMe)(3)(TMT). J. Organomet. Chem. 2002, 645, 101–104. [Google Scholar] [CrossRef]
- Haiduc, I.; Mahon, M.F.; Molloy, K.C.; Venter, M.M. Synthesis and spectral characterisation of organotin(IV) 1,3,5-triazine-2,4,6-trithiolato complexes, including the crystal structures of 1,3,5-(R3Sn)(3)C3N3S3 (R = Me, Ph). J. Organomet. Chem. 2001, 627, 6–12. [Google Scholar] [CrossRef]
- Chan, C.K.; Cheung, K.K.; Che, C.M. Structure and spectroscopic properties of a luminescent inorganic cyclophane from self-assembly of copper(I) and two ligand components. Chem. Commun. 1996, 1996, 227–228. [Google Scholar] [CrossRef]
- Tzeng, B.C.; Che, C.M.; Peng, S.M. Luminescent gold(I) supermolecules with trithiocyanuric acid. Crystal structure, spectroscopic and photophysical properties. Chem. Commun. 1997, 1997, 1771–1772. [Google Scholar] [CrossRef]
- Li, D.; Shi, W.J.; Hou, L. Coordination polymers of copper(I) halides and neutral heterocyclic thiones with new coordination modes. Inorg. Chem. 2005, 44, 3907–3913. [Google Scholar] [CrossRef]
- Trivedi, M.; Pandey, D.S.; Zou, R.Q.; Xu, Q. Novel Rh(III) pentaryiethylcyclopentadienyl and Ru(II) cyclopentadienyl complexes containing 1,3,5-triazine-2,4,6-trithiol in trinucleating mode. Inorg. Chem. Commun. 2008, 11, 526–530. [Google Scholar] [CrossRef]
- Han, Y.F.; Huang, Y.B.; Lin, Y.H.; Jin, G.X. Synthesis, characterization, and norbornene polymerization behavior of the half-sandwich complexes CP*(3)M(3)(mu(3)-L)Cl(3) and Cp*M(2-SPyH)Cl(2) (M = Ir, M = Rh, L (3-) = 1,3,5-triazine-2,4,6-trithiolato, 2-SPy = 2-pyridinethione). Organometallics 2008, 27, 961–966. [Google Scholar] [CrossRef]
- Prushan, M.J.; Privette, N.K.; Zeller, M.; Hunter, A.D.; Lofland, S.; Preite, S.D. Synthesis, characterization and reactivity of a trinuclear copper(II) thiocyanurate complex: A spin-frustrated molecular propeller. Inorg. Chem. Commun. 2007, 10, 631–635. [Google Scholar] [CrossRef]
- Marek, J.; Travnicek, Z.; Cermakova, S. (mu(3)-Trithiocyanurato-kappa(6) N-1, S-2 : N-3, S-4 : N-5, S-6) tris (N, N, N', N'', N''-pentamethyldiethylenetriamine-kappa(3) N, N', N'') zinc(II) tris(perchlorate). Acta Crystallogr. Sect. E.-Struct Rep. Online 2007, 63, M1411–M1413. [Google Scholar]
- Kopel, P.; Cermakova, S.; Dolezal, K.; Kalinska, B.; Bienko, A.; Mrozinski, J. Synthesis and properties of a trinuclear copper(II) complex with trithiocyanurate bridge. Pol. J. Chem. 2007, 81, 327–335. [Google Scholar]
- Travnicek, Z.; Marek, J.; Cermakova, S. (mu(3)-Trithiocyanurato-kappa N-6(1), S-2 : N-3, S-4 : N-5, S-6) tris (N, N, N', N'', N''-pentamethyldiethylenetriamine-kappa N-3, N', N'')copper(II) tris(perchlorate). Acta Crystallogr. Sect. E.-Struct Rep. Online 2007, 63, M1742–U1430. [Google Scholar] [CrossRef]
- Kopel, P.; Mrozinski, J.; Dolezal, K.; Langer, V.; Boca, R.; Bienko, A.; Pochaba, A. Ferromagnetic Properties of a Trinuclear Nickel(II) Complex with a Trithiocyanurate Bridge. Eur. J. Inorg. Chem. 2009, 2009, 5475–5482. [Google Scholar]
- Musilek, K.; Dolezal, M.; Gunn-Moore, F.; Kuca, K. Design, Evaluation and Structure-Activity Relationship Studies of the AChE Reactivators Against Organophosphorus Pesticides. Med. Res. Rev. 2011, 31, 548–575. [Google Scholar] [CrossRef]
- Johansso, L.; Larsson, R.; Blomquis, J.; Cederstr, C.; Grapengi, S.; Helgeson, U.; Moberg, L.C.; Sundbom, M. X-ray photoelectron and mossbauer-spectroscopy on a variety of iron compounds. Chem. Phys. Lett. 1974, 24, 508–513. [Google Scholar] [CrossRef]
- Sato, H.; Tominaga, T. Mossbauer studies of thermal-decomposition of tris(2,2´-bipyridine)iron(II) chloride and structures of isomers of 2,2´-bipyridineiron(II) chloride. Bull. Chem. Soc. Jpn. 1976, 49, 697–700. [Google Scholar] [CrossRef]
- Cermakova, S.; Herchel, R.; Travnicek, Z.; Sebela, M. Syntheses and magnetic properties of trinuclear trithiocyanurato-bridged manganese(II) complexes involving bidentate aromatic N-donor heterocycles. Inorg. Chem. Commun. 2010, 13, 778–781. [Google Scholar] [CrossRef]
- Comba, P.; Lampeka, Y.D.; Lotzbeyer, L.; Prikhod'ko, A.I. Macrocyclic melamine-based ligand complexes as building blocks for the metal-directed synthesis of heterometallic di- and trinuclear compounds. Eur. J. Inorg. Chem. 2003, 2003, 34–37. [Google Scholar] [CrossRef]
- Pavlishchuk, V.V.; Kolotilov, S.V.; Addison, A.W.; Prushan, M.J.; Butcher, R.J.; Thompson, L.K. Mono- and trinuclear nickel(II) complexes with sulfur-containing oxime ligands: Uncommon templated coupling of oxime with nitrile. Inorg. Chem. 1999, 38, 1759–1766. [Google Scholar] [CrossRef]
- Dvorak, Z.; Starha, P.; Sindelar, Z.; Travnicek, Z. Evaluation of in vitro cytotoxicity of one-dimensional chain Fe(salen)(L) (n) complexes against human cancer cell lines. Toxicol. In Vitro 2012, 26, 480–484. [Google Scholar] [CrossRef]
- Aoki, S.; Zulkefeli, M.; Shiro, M.; Kimura, E. New supramolecular trigonal prisms from zinc(II)-1,4,7,10-tetraazacyclododecane (cyclen) complexes and trithiocyanurate in aqueous solution. Proc. Natl. Acad. Sci. USA 2002, 99, 4894–4899. [Google Scholar] [CrossRef]
- SMART-SAINT. Area Detector Control and Integration Software. Bruker AXS Inc.: Madison, WI, USA, , 2003. [Google Scholar]
- SADABS. Program for Empirical Absorption Correction for Area Detectors (Version 2.10). Sheldrick, G.M., Ed.; University of Gottingen: Gottingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Kuca, K.; Cabal, J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin nerve agent. Toxicol. Mech. Methods 2005, 15, 247–252. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the prepared compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kopel, P.; Dolezal, K.; Langer, V.; Jun, D.; Adam, V.; Kuca, K.; Kizek, R. Trithiocyanurate Complexes of Iron, Manganese and Nickel and Their Anticholinesterase Activity. Molecules 2014, 19, 4338-4354. https://doi.org/10.3390/molecules19044338
Kopel P, Dolezal K, Langer V, Jun D, Adam V, Kuca K, Kizek R. Trithiocyanurate Complexes of Iron, Manganese and Nickel and Their Anticholinesterase Activity. Molecules. 2014; 19(4):4338-4354. https://doi.org/10.3390/molecules19044338
Chicago/Turabian StyleKopel, Pavel, Karel Dolezal, Vratislav Langer, Daniel Jun, Vojtech Adam, Kamil Kuca, and Rene Kizek. 2014. "Trithiocyanurate Complexes of Iron, Manganese and Nickel and Their Anticholinesterase Activity" Molecules 19, no. 4: 4338-4354. https://doi.org/10.3390/molecules19044338