An Unusual 2,3-Secotaraxerene and Other Cytotoxic Triterpenoids from Pleiocarpa pycnantha (Apocynaceae) Leaves Collected from Nigeria
Abstract
:1. Introduction
2. Results and Discussion
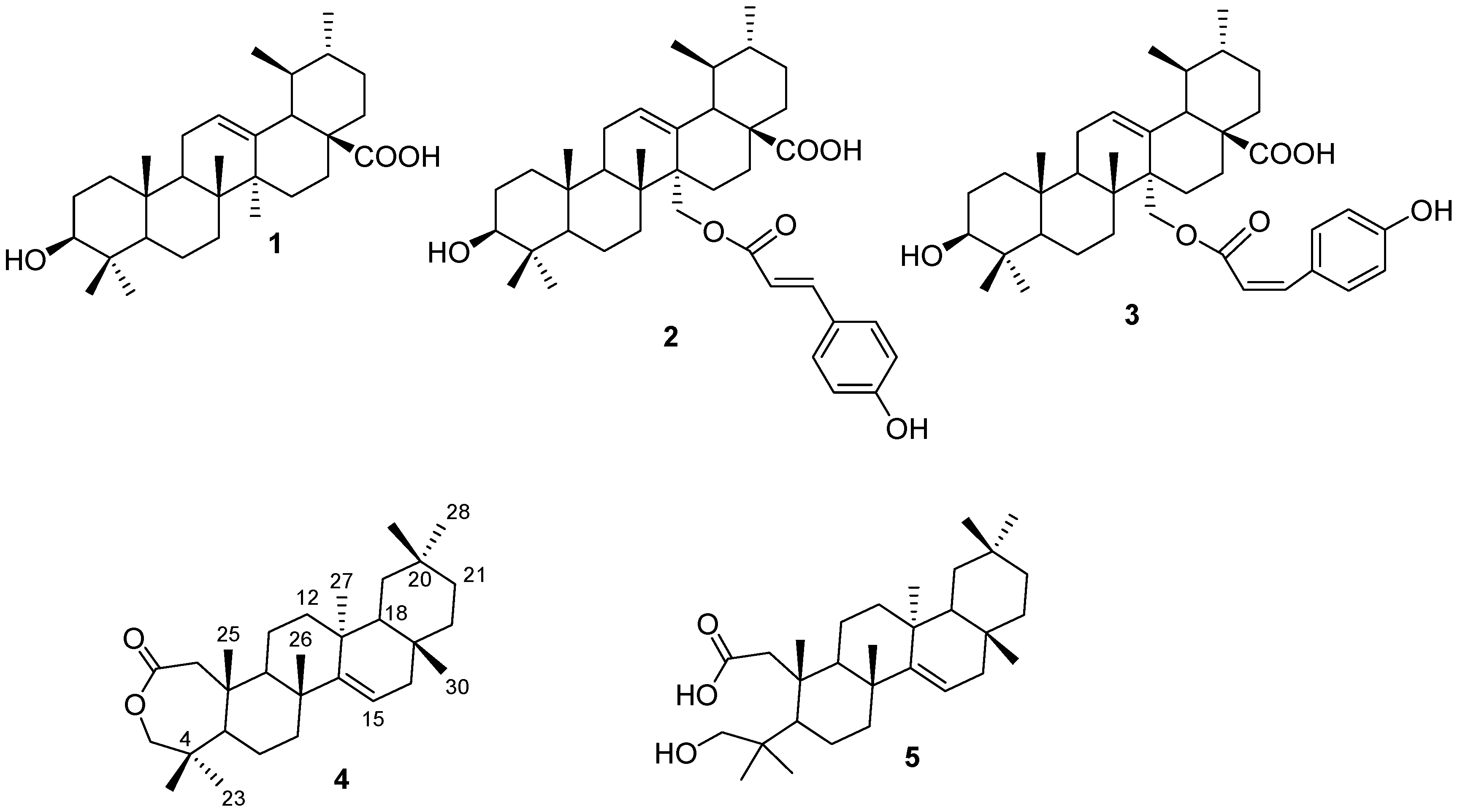

| Position | Compound 4 | Compound 5 | ||
|---|---|---|---|---|
| 13C | 1H (J Hz) | 13C | 1H (J Hz) | |
| 1 | 44.9 t | 2.44 br s; 2.78 br s | 43.1 t | 2.36 d, 2.35 d, (12.0) |
| 2 | 174.8 s | ----- | 172.9 s | ----- |
| 3 | 77.9 t | 3.79 br s; 4.08 br s | 72.1 t | 3.33 d; 3.34 d, (7.0) |
| 4 | 38.4 s | ----- | 38.6 s | ----- |
| 5 | 48.7 d | 0.97 * | 44.9 d | 1.72 * |
| 6 | 20.6 t | 1.57 *; 1.66 * | 21.8 t | 1.60 *; 1.53 * |
| 7 | 35.1 t | 1.02 *; 1.35 * | 35.6 t | 0.95 *; 1.34 * |
| 8 | 35.8 s | ----- | 34.9 s | ----- |
| 9 | 40.9 d | 1.64 * | 42.8 d | 1.76 * |
| 10 | 46.7 s | ----- | 43.6 s | ----- |
| 11 | 18.6 t | 1.57 *; 1.75 br s | 29.5 t | 1.18 * (2H) |
| 12 | 36.6 t | 0.98 (dd; 12.0, 4.5); 1.34 * | 37.5 t | 1.49 *; 1.83 (td; 9.0, 3.5) |
| 13 | 37.4 s | ----- | 37.0 s | ----- |
| 14 | 157.5 s | ----- | 157.3 s | ----- |
| 15 | 117.1 d | 5.54 (dd; 8.0, 3.5) | 116.8 d | 5.46 dd, (9.0, 4.5) |
| 16 | 37.8 t | 1.64 *; 1.92 (dd; 9.0, 2.0) | 36.5 t | 0.90 *; 1.22 * |
| 17 | 40.6 s | ----- | 40.2 s | ----- |
| 18 | 48.7 d | 0.97 * | 48.5 d | 0.92 * (2H) |
| 19 | 39.4 t | 1.37 *; 2.04 (dt; 9.5, 3.5) | 39.7 t | 1.33*; 1.96 (dd; 3.5, 8.5) |
| 20 | 28.7*s | ----- | 28.6 s | ----- |
| 21 | 33.1 t | 1.25 (dt; 9.5, 3.2); 1.32 * | 32.9 t | 1.44 *; 1.59 * |
| 22 | 33.6 t | 1.60 *; 1.64 * | 33.8 t | 1.55 *; 1.60 * |
| 23 | 19.4 q | 1.02 s | 26.3 s | 0.93 s |
| 24 | 28.7 q | 0.95 s | 24.4d | 1.04 s |
| 25 | 17.5 q | 1.13 s | 18.5 q | 1.02 s |
| 26 | 25.8 q | 1.11 s | 20.6 q | 0.86 s |
| 27 | 29.6 q | 0.92 s | 29.5 q | 0.83 s |
| 28 | 21.1 q | 0.90 s | 23.9 q | 1.00 s |
| 29 | 33.5 q | 0.95 s | 33.1 q | 0.87 s |
| 30 | 29.6 q | 0.83 s | 29.7 q | 0.71 s |
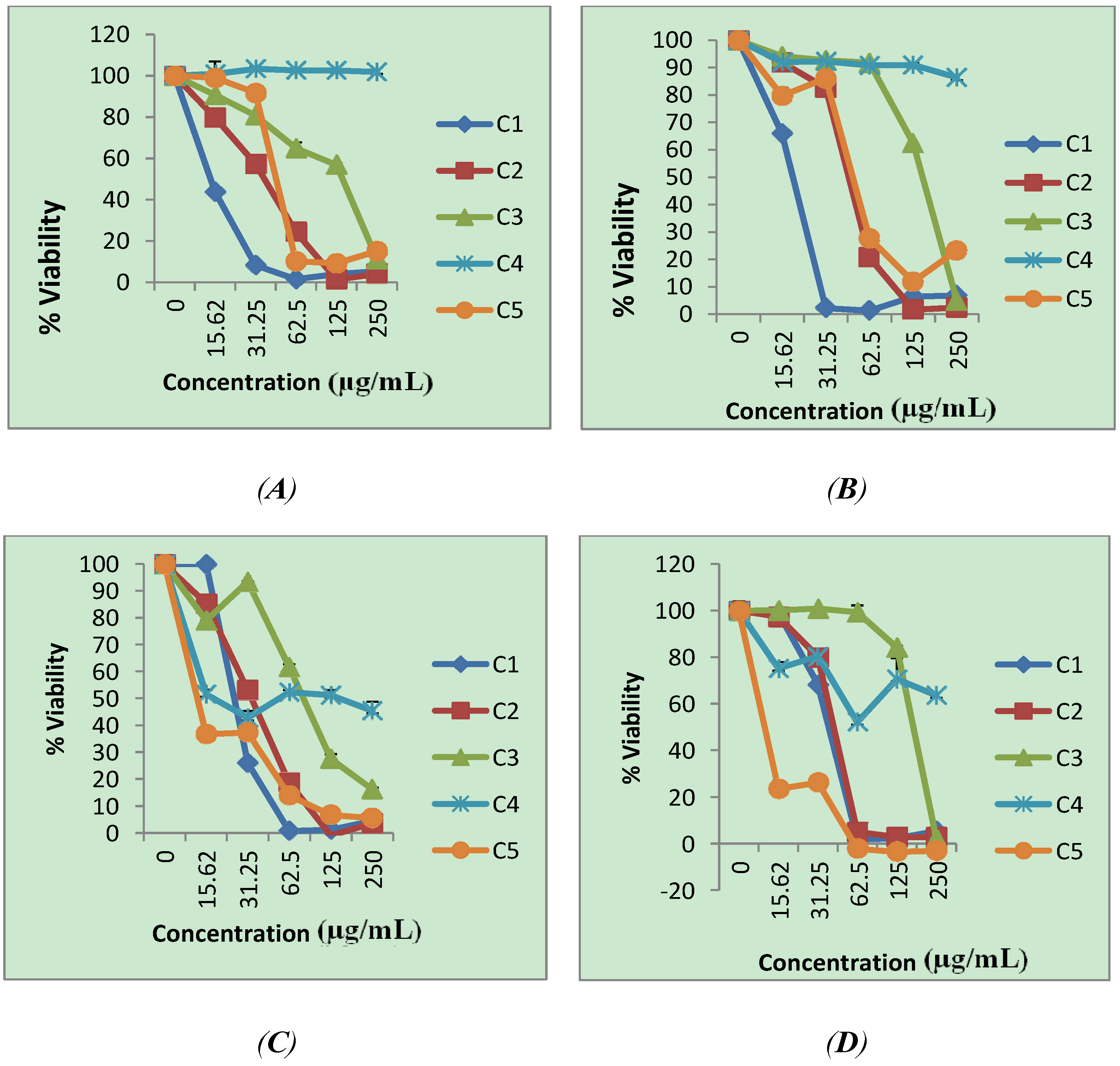
| Compound | Cell lines | ||||||
|---|---|---|---|---|---|---|---|
| HeLa | HT-29 | MCF-7 | KMST-6 | ||||
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| 1 | 10 | 10.0 | 10 | 10.0 | 20 | 5.0 | 100 |
| 2 | 50 | 2 | 100 | 1 | 60 | 1.7 | 100 |
| 3 | 200 | 1.5 | 230 | 1.3 | 180 | 1.7 | 300 |
| 4 | >600 | ----- | >600 | ----- | 20 | >30 | >600 |
| 5 | 180 | 0.06 | 170 | 0.06 | 10 | 1 | 10 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectra Data
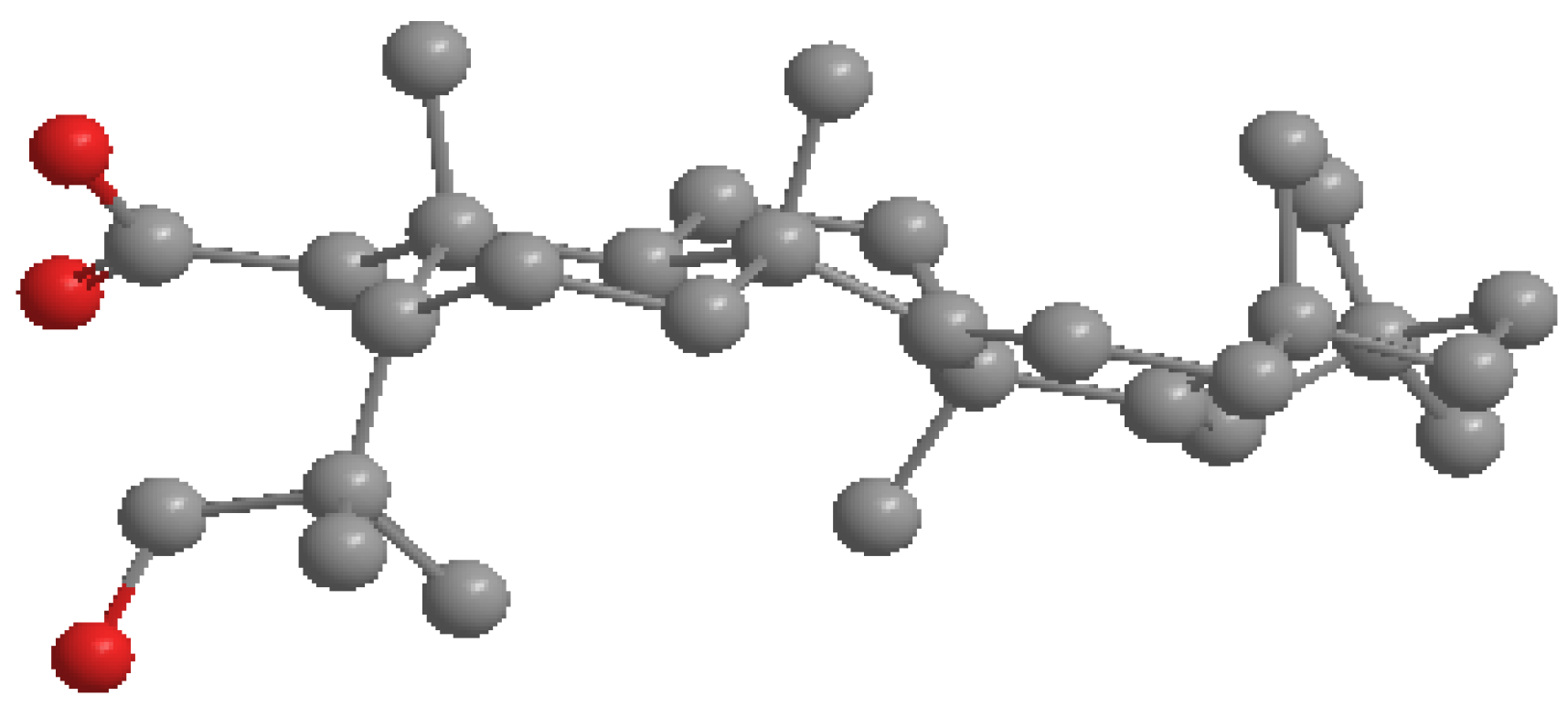
3.5. X-ray Structure Determination of 4
3.6. Culture of Cell Lines
3.7. WST-1 Based Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Trease, G.E.; Evans, W.C. Pharmacognosy, 14th ed.; W.B. Saunders: London, UK, 1996. [Google Scholar]
- Samuelsson, G. Drugs of Natural Origin. A Textbook of Pharmacognosy; Swedish Pharmaceutical Press: Stockholm, Sweden, 2004. [Google Scholar]
- Brouwer, N.; Liu, Q.; Harrington, D.; Kohen, J.; Vemulpad, S.; Jamie, J.; Randall, M.; Randall, D. An ethnopharmacological study of medicinal plants in New South Wales. Molecules 2005, 10, 1252–1262. [Google Scholar] [CrossRef]
- Adebayo, J.O.; Krettli, A.U. Potential antimalarials from Nigerian plants: A review. J. Ethnopharmacol. 2011, 133, 289–302. [Google Scholar] [CrossRef]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Traditional Medicine. Available online: http://www.who.int/topics/traditional_medicine/en/ (accessed on 25 March 2013).
- Le Grand, A.; Wondergem, P. Herbal Medicine and Health Promotion. A Comparative Study of Herbal Drugs in Primary Health Care; Royal Tropical Institute: Amsterdam, The Netherlands, 1989; pp. 7–20. [Google Scholar]
- Cordell, G.A. Changing strategies in natural products chemistry. Phytochemistry 1995, 40, 1585–1612. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer Statistics 2012. Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants Used Against Cancer; Quarterman: Lawrence, MA, USA, 1982. [Google Scholar]
- Cragg, G.M.; Kingston, D.G.I.; Newman, D.J. (Eds.) Anticancer Agents from Natural Products; Brunner-Routledge Psychology Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005.
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of Asia and the Pacific; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gan, L.S.; Yang, S.P.; Wu, Y.; Ding, J.; Yue, J.M. Terpenoid indole alkaloids from Winchia calophylla. J. Nat. Prod. 2006, 69, 18–22. [Google Scholar] [CrossRef]
- Lim, S.H.; Sim, K.M.; Abdullah, Z.; Hiraku, O.; Hayashi, M.; Komiyama, K.; Kam, T.S. Leuconoxine, kopsinitarine, kopsijasmine, and kopsinone derivatives from Kopsia. J. Nat. Prod. 2007, 70, 1380–1383. [Google Scholar] [CrossRef]
- Wang, G.C.; Zhong, X.Z.; Zhang, D.M. Two pairs of epimeric indole alkaloids from Catharanthus roseus. Planta Med. 2011, 77, 1739–1741. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Vidar, W.S.; Chen, X.; Decker, M.; Wan, J.H.B.; Franzblau, S.G.; Galvez, E.V.; Aguinaldo, M.A.M.; Cordell, G.A. Mycobacterium tuberculosis and cholinesterase inhibitors from Voacanga globosa. Eur. J. Med. Chem. 2011, 46, 3118–3123. [Google Scholar] [CrossRef]
- Aremu, A.O.; Finnie, J.F.; van Staden, J. Potential of South African medicinal plants used as anthelmintics—Their efficacy, safety concerns and reappraisal of current screening methods. S. Afr. J. Bot. 2012, 82, 143–150. [Google Scholar]
- Burkill, H.M. The Useful Plants of West Tropical Africa 2nd Edition Volume 1, Families A–D; Royal Botanic Gardens: Kew, Richmond, UK, 1985; p. 960. [Google Scholar]
- Fatumbi, V.P. Ewé: The Use of Plants in Yoruba Society; Editora Schwarcz LTDA: Sao Paulo, PB, Brazil, 1995. [Google Scholar]
- Gorman, A.A.; Schmid, H. Structure of dimeric indole alkaloid pycnanthinine. Monatsh. Chem. 1967, 98, 1554–1566. [Google Scholar] [CrossRef]
- Gorman, A.A.; Dastoor, N.J.; Manfred, H.; von Philipsborn, W.; Renner, U.; Schmid, H. Alkaloids. CXXXII. Constitution of two new type dimeric indole alkaloids, pycnanthine and pleiomutinine. Helv. Chim. Acta 1969, 52, 33–55. [Google Scholar] [CrossRef]
- Keawpradub, N.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J. Activity of extracts and alkaloids of Thai Alstonia species against human lung cancer cell lines. Planta Med. 1997, 63, 97–101. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Siddiqui, S.; Siddiqui, B.S.; Naeed, A.; Begum, S. Three pentacyclic triterpenoids from the leaves of Plumeria obtusa. J. Nat. Prod. 1990, 53, 1332–1336. [Google Scholar] [CrossRef]
- Budzikiewicz, H.; Thomas, H. p-Cumaroxy-ursolsaure, ein neuer inhaltstoff von Ilex aquifolium. L. Z. Naturforsch. 1980, 35, 230–231. [Google Scholar]
- Danylec, B.; Iskander, M. 1H-NMR measurement of the trans-cis photoisomerization of cinnamic acid derivatives. J. Chem. Ed. 2002, 79, 1000–1001. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C-NMR Spectra of pentacyclic triterpenoids—A complication and some salient features. Phytochemistry 1994, 37, 1517–1573. [Google Scholar] [CrossRef]
- Tu, H.Y.; Huang, A.M.; Wei, B.L.; Gan, K.H.; Hour, T.C.; Pu, Y.S.; Lin, C.N. Ursolic acid derivatives induce cell cycle arrest and apoptosis in NTUB1 cells associated with reactive oxygen species. Bioorg. Med. Chem. 2009, 17, 7265–7274. [Google Scholar] [CrossRef]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Media Centre. Available online: http://www.who.int/mediacentre/news/releases/2003/pr27/en/ (accessed on 1 July 2013).
- Silva, J.A.; Silva, A.G.; Alves, A.S.; Reis, R.; Nascimento, C.C.; Diré, G.F.; Barreto, A.S. Plumeria rubra (Apocynaceae): A good source of ursolic acid. J. Med. Plants Res. 2013, 7, 892–896. [Google Scholar]
- Nishimura, K.; Fukuda, T.; Miyase, T.; Noguchi, H.; Chen, X.M. Activity-guided isolation of triterpenoid acyl CoA cholesteryl acyl transferase (ACAT) inhibitors from Ilex kudincha. J. Nat. Prod. 1999, 62, 1061–1064. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar]
- Kondo, M. Phytochemical Studies of Extracts from Cranberry (Vaccinium macrocarpon) with Anti-Cancer, Anti-Fungal and Cardioprotective Properties. M.S. Thesis, University of Massachusetts, Massachusetts, MA, USA, 2006. [Google Scholar]
- Hamzah, A.S.; Lajis, N.H. Chemical constituents of Hedyotis herbacea. ARBEC. 1998. article II. Available online: http://www.arbec.com.my/pdf/may-2.pdf (accessed on 11 March 2014).
- Lee, J.S.; Kim, J.; Kim, B.Y.; Lee, H.S.; Ahn, J.S.; Chang, Y.S. Inhibition of Phospholipase Cγ1 and Cancer Cell Proliferation by Triterpene Esters from Uncaria rhynchophylla. J. Nat. Prod. 2000, 63, 753–756. [Google Scholar] [CrossRef]
- Okouneva, T.; Hill, B.T.; Wilson, L.; Jordan, M.A. The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Mol. Cancer Ther. 2003, 2, 427–436. [Google Scholar]
- Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary crystallographic data (CCDC 972299) for 4 and can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- Bruker. SAINT Version 7.60a; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, SHELXL-97 and SADABS Version 2.05; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Atwood, J.L.; Barbour, L.J. Molecular Graphics: From Science to Art. Cryst. Growth Des. 2003, 3, 3–8. [Google Scholar] [CrossRef]
- POV-Ray for Windows (Version 3.6). Persistence of Vision Raytracer Pty. Ltd.: Victoria, Australia. Available online: http://www.povray.org (accessed on 12 March 2004).
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Omoyeni, O.A.; Meyer, M.; Iwuoha, E.; Green, I.; Hussein, A.A. An Unusual 2,3-Secotaraxerene and Other Cytotoxic Triterpenoids from Pleiocarpa pycnantha (Apocynaceae) Leaves Collected from Nigeria. Molecules 2014, 19, 3389-3400. https://doi.org/10.3390/molecules19033389
Omoyeni OA, Meyer M, Iwuoha E, Green I, Hussein AA. An Unusual 2,3-Secotaraxerene and Other Cytotoxic Triterpenoids from Pleiocarpa pycnantha (Apocynaceae) Leaves Collected from Nigeria. Molecules. 2014; 19(3):3389-3400. https://doi.org/10.3390/molecules19033389
Chicago/Turabian StyleOmoyeni, Olubunmi A., Mervin Meyer, Emmanuel Iwuoha, Ivan Green, and Ahmed A. Hussein. 2014. "An Unusual 2,3-Secotaraxerene and Other Cytotoxic Triterpenoids from Pleiocarpa pycnantha (Apocynaceae) Leaves Collected from Nigeria" Molecules 19, no. 3: 3389-3400. https://doi.org/10.3390/molecules19033389






