Synthesis of Novel Highly Functionalized 4-Thiazolidinone Derivatives from 4-Phenyl-3-thiosemicarbazones
Abstract
:1. Introduction
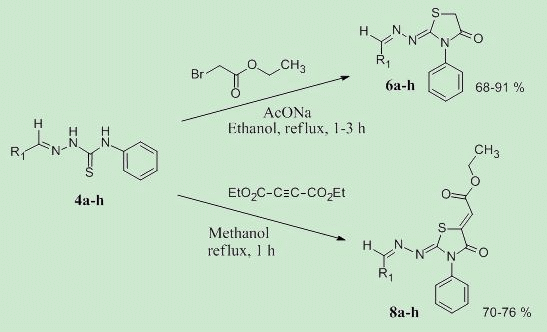
2. Results and Discussion

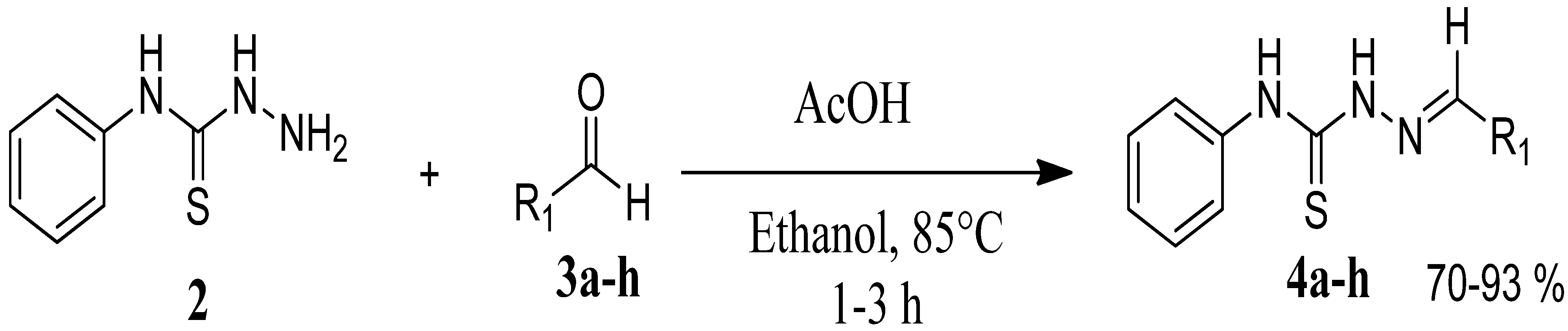
| Carbonyl compound | Product | Product number | Reaction time (h) | Yield (%) |
|---|---|---|---|---|
 |  | 4a | 1 | 70 |
 |  | 4b | 3 | 90 |
 |  | 4c | 2 | 93 |
 |  | 4d | 1 | 90 |
 |  | 4e | 1 | 91 |
 |  | 4f | 2 | 89 |
 |  | 4g | 2 | 91 |
 |  | 4h | 1 | 89 |
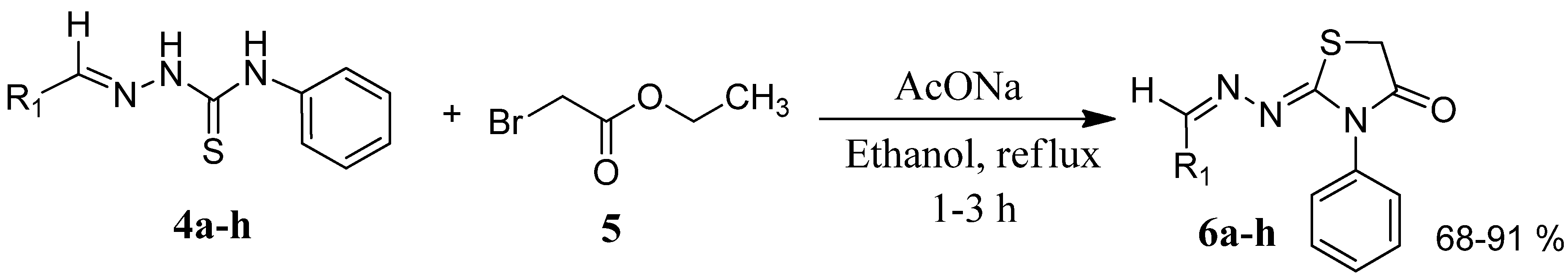
| Compound | Product | Product number | Reaction time (h) | Yield (%) |
|---|---|---|---|---|
| 4a |  | 6a | 1 | 91 |
| 4b |  | 6b | 3 | 68 |
| 4c |  | 6c | 3 | 82 |
| 4d |  | 6d | 2 | 86 |
| 4e |  | 6e | 2 | 80 |
| 4f |  | 6f | 3 | 89 |
| 4g |  | 6g | 3 | 90 |
| 4h |  | 6h | 2 | 88 |
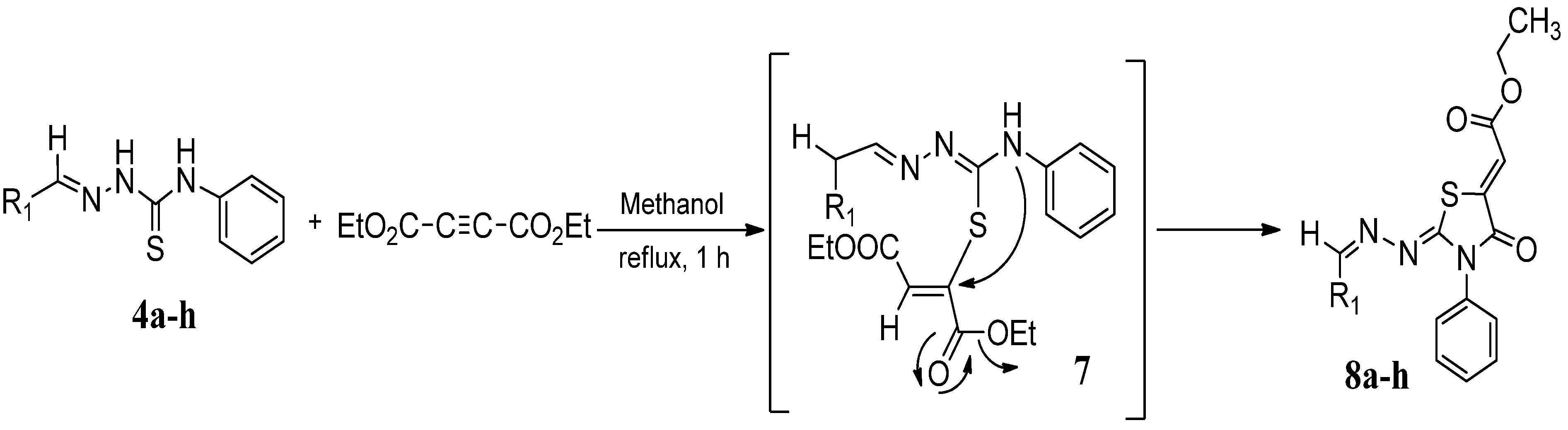
| Compound | Product | Product number | Yield (%) |
|---|---|---|---|
| 4a |  | 8a | 73 |
| 4b |  | 8b | 70 |
| 4c |  | 8c | 76 |
| 4d |  | 8d | 74 |
| 4e |  | 8e | 73 |
| 4f |  | 8f | 75 |
| 4g |  | 8g | 70 |
| 4h |  | 8h | 71 |
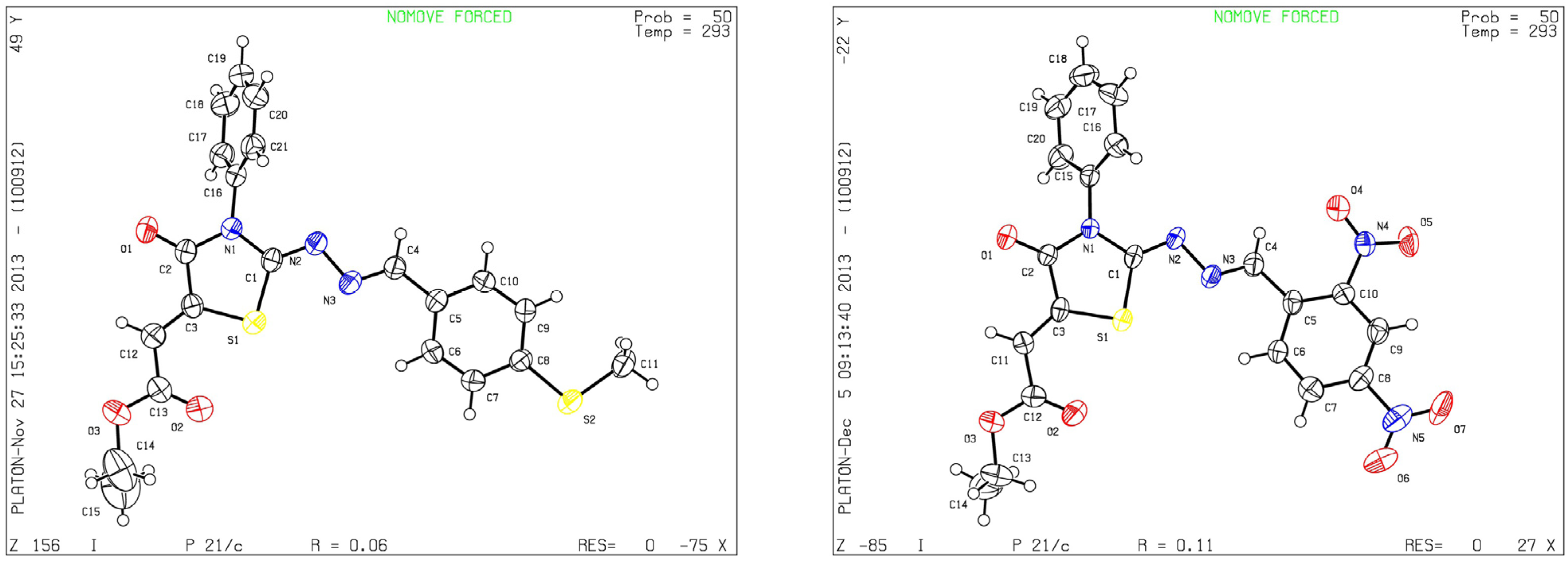
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of Compounds 4a–h
3.3. General Procedure for the Preparation of Compounds 6a–h
3.4. General Procedure for the Preparation of Compounds 8a–h
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References and Notes
- Pirrung, M.C.; Pansare, S.V.; das Sarma, K.; Keith, K.A.; Kern, E.R. Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents. J. Med. Chem. 2005, 48, 3045–3050. [Google Scholar] [CrossRef]
- Hu, W.-X.; Zhou, W.; Xia, C.-N.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2006, 16, 2213–2218. [Google Scholar] [CrossRef]
- Kolocouris, A.; Dimas, K.; Pannecouque, C.; Witvrouw, M.; Foscolos, G.B.; Stamatiou, G.; Fytas, G.; Zoidis, G.; Kolocouris, N.; Andrei, G.; et al. New 2-(1-adamantylcarbonyl) pyridine and 1-acetyladamantane thiosemicarbazones-thiocarbonohydrazones: Cell growth inhibitory, antiviral and antimicrobial activity evaluation. Bioorg. Med. Chem. Lett. 2002, 12, 723–727. [Google Scholar] [CrossRef]
- Tarasconi, P.; Capacchi, S.; Pelosi, G.; Cornia, M.; Albertini, R.; Bonati, A.; Dall’Aglio, P.P.; Lunghi, P.; Pinelli, S. Synthesis, spectroscopic characterization and biological properties of new natural aldehydes thiosemicarbazones. Bioorg. Med. Chem. 2000, 88, 157. [Google Scholar]
- Pérez-Rebolledo, A.; Teixeira, L.R.; Batista, A.A.; Mangrich, A.S.; Aguirre, G.; Cerceretto, H.; González, M.; Hernández, P.; Ferreira, A.M.; Speziali, N.L.; et al. 4-Nitroacetophenone-derived thiosemicarbazones and their copper (II) complexes with significant in vitro anti-trypanosomal activity. Eur. J. Med. Chem. 2008, 43, 939–948. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; et al. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef]
- Du, X.; Guo, C.; Hansell, E.; Doyle, P.S.; Caffrey, C.R.; Holler, T.P.; McKerrow, J.H.; Cohen, E. Synthesis and structure-activity relationship study of potent trypanocidal thio- semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 2002, 45, 2695–2707. [Google Scholar] [CrossRef]
- De Aquino, T.M.; Liesen, A.P.; da Silva, R.E.A.; Lima, V.T.; Carvalho, C.S.; de Faria, A.R.; de Araujo, J.M.; de Lima, J.G.; Alves, A.J.; de Melo, E.J.T.; et al. Synthesis, anti-Toxoplasma-gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenymethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidine acetic acids. Bioorg. Med. Chem. 2008, 16, 446–456. [Google Scholar] [CrossRef]
- Mayekar, S.A.; Mulwad, V.V. Synthesis and antibacterial activity of 6-(5phenyl-{1,3,4} thiadiazol-2-ylimino)-benzopyran-2-ones. Ind. J. Chem. 2008, 47, 1438–1442. [Google Scholar]
- Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Sokovic, M.; Ciric, A.; Glamoclija, J. Novel 4- thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010, 18, 426–432. [Google Scholar] [CrossRef]
- Bhat, M.A.; Siddiqui, N.; Khan, S.A. Synthesis, anticonvulsant and neurotoxicity screening of 2-(substituted phenyl)-3-[3-(2-oxo-2H-chromen-3-yl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-1,3-thiazolidin-4-ones. Ind. J. Het. Chem. 2008, 17, 287–288. [Google Scholar]
- Babaoglu, K.; Page, M.A.; Jones, V.C.; McNeil, M.R.; Dong, C.; Naismith, J.H.; Lee, R.E. Novel inhibitors of an emerging target in mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg. Med. Chem. Lett. 2003, 13, 3227–3230. [Google Scholar] [CrossRef]
- Vigorita, M.G.; Ottanà, R.; Monforte, F.; Maccari, R.; Monforte, M.T.; Trovato, A.; Taviano, M.F.; Miceli, N.; de Luca, G.; Alcaro, S.; et al. Chiral 3,3'-(1,2-ethanediyl)-bis[2-(3,4-dimethoxyphenyl)-4-thiazolidinones] with anti-inflammatory activity. Part 11: Evaluation of COX-2 selectivity and modeling. Bioorg. Med. Chem. 2003, 11, 999–1006. [Google Scholar] [CrossRef]
- Agrawal, V.K.; Sachan, S.; Khadikar, P.V. QSAR studies on antihistaminic activity of some thiazolidine-4-ones. Acta Pharm. 2000, 50, 281–290. [Google Scholar]
- Diurno, M.V.; Mazzoni, O.; Correale, G.; Monterrey, I.G.; Calignano, A.; la Rana, G.; Bolognese, A. Synthesis and structure-activity relationships of 2-(substituted phenyl)-3-[3-(N,N-dimethylamino)-propyl]-1,3-thiazolidin-4-ones acting as H1-histamine antagonists. Farmaco 1999, 54, 579–583. [Google Scholar] [CrossRef]
- Suzuki, Y.; Akima, M.; Tamura, K. Effects of CP-060S, a novel cardioprotective drug, on cardiac function and myocardial oxygen consumption. Gen. Pharmacol. 1999, 32, 57–63. [Google Scholar] [CrossRef]
- Rawal, R.K.; Prabhakar, Y.S.; Katti, S.B.; de Clercq, E. 2-(Aryl)-3-furan-2-ylmethyl-thiazolidin-4-ones as selective HIV-RT inhibitors. Bioorg. Med. Chem. 2005, 13, 6771–6776. [Google Scholar] [CrossRef]
- Crozet, M.P.; Archaimbault, G.; Vanelle, P.; Nouguier, R. Reaction SRN1 en serie heterocyclique: Reactivite des sels du dimethyl-2,2-nitro-5-dioxane-1,3. Tetrahedron Lett. 1985, 26, 5133–5134. [Google Scholar] [CrossRef]
- Amiri-Attou, O.; Terme, T.; Vanelle, P. Functionalization of 6-nitrobenzo[1,3]dioxole with carbonyl compounds via TDAE methodology. Molecules 2005, 10, 545–551. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Hutter, S.; Castera-Ducros, C.; Laget, M.; Dumètre, A.; Gasquet, M.; Reboul, J.P.; Rault, S.; Rathelot, P.; et al. Synthesis and in vitro antiplasmodial evaluation of 4-anilino-2-trichloromethylquinazolines. Bioorg. Med. Chem. 2009, 17, 4313–4322. [Google Scholar] [CrossRef]
- Crozet, M.D.; Botta, C.; Gasquet, M.; Curti, C.; Remusat, V.; Hutter, S.; Chapelle, O.; Azas, N.; de Méo, M.; Vanelle, P. Lowering of 5-nitroimidazole’s mutagenicity: Towards optimal antiparasitic pharmacophore. Eur. J. Med. Chem. 2009, 44, 653–659. [Google Scholar] [CrossRef]
- Kabri, Y.; Azas, N.; Dumètre, A.; Hutter, S.; Laget, M.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Original quinazoline derivatives displaying antiplasmodial properties. Eur. J. Med. Chem. 2010, 45, 616–622. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Q.; Ku, X.; Meng, L.; Lin, L.; Wang, X.; Zhu, C.; Wang, Y.; Chen, Z.; Li, M.; et al. A series of α-heterocyclic carboxaldehyde thiosemicarbazones inhibit topoisomerase IIα catalytic activity. J. Med. Chem. 2010, 53, 3048–3064. [Google Scholar] [CrossRef]
- Serda, M.; Malecki, J.G.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Polanski, J. Microwave assisted synthesis, X-ray crystallography and DFT calculations of selected aromatic thiosemicarbazones. J. Mol. Struct. 2013, 1037, 63–72. [Google Scholar] [CrossRef]
- Küçügüzel, G.; Kocayepe, A.; de Clercq, E.; Sahin, F.; Güllüce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar] [CrossRef]
- Vas’kevich, R.I.; Zborovskii, Y.L.; Staninets, V.I.; Chernega, A.N. Reaction of 4-Aryl-1-(4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-2-yl)thiosemicarbazides with Dimethyl Acetylenedicarboxylate. Russ. J. Org. Chem. 2004, 40, 1047–1052. [Google Scholar] [CrossRef]
- CCDC contains the supplementary crystallographic data of compound 8b for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- CCDC contains the supplementary crystallographic data of compound 8g for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- Sample Availability: Samples of the compounds 4a–h, 6a–h and 8a–h are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Benmohammed, A.; Khoumeri, O.; Djafri, A.; Terme, T.; Vanelle, P. Synthesis of Novel Highly Functionalized 4-Thiazolidinone Derivatives from 4-Phenyl-3-thiosemicarbazones. Molecules 2014, 19, 3068-3083. https://doi.org/10.3390/molecules19033068
Benmohammed A, Khoumeri O, Djafri A, Terme T, Vanelle P. Synthesis of Novel Highly Functionalized 4-Thiazolidinone Derivatives from 4-Phenyl-3-thiosemicarbazones. Molecules. 2014; 19(3):3068-3083. https://doi.org/10.3390/molecules19033068
Chicago/Turabian StyleBenmohammed, Abdelmadjid, Omar Khoumeri, Ayada Djafri, Thierry Terme, and Patrice Vanelle. 2014. "Synthesis of Novel Highly Functionalized 4-Thiazolidinone Derivatives from 4-Phenyl-3-thiosemicarbazones" Molecules 19, no. 3: 3068-3083. https://doi.org/10.3390/molecules19033068