Effects of Platycodin D on Proliferation, Apoptosis and PI3K/Akt Signal Pathway of Human Glioma U251 Cells
Abstract
:1. Introduction
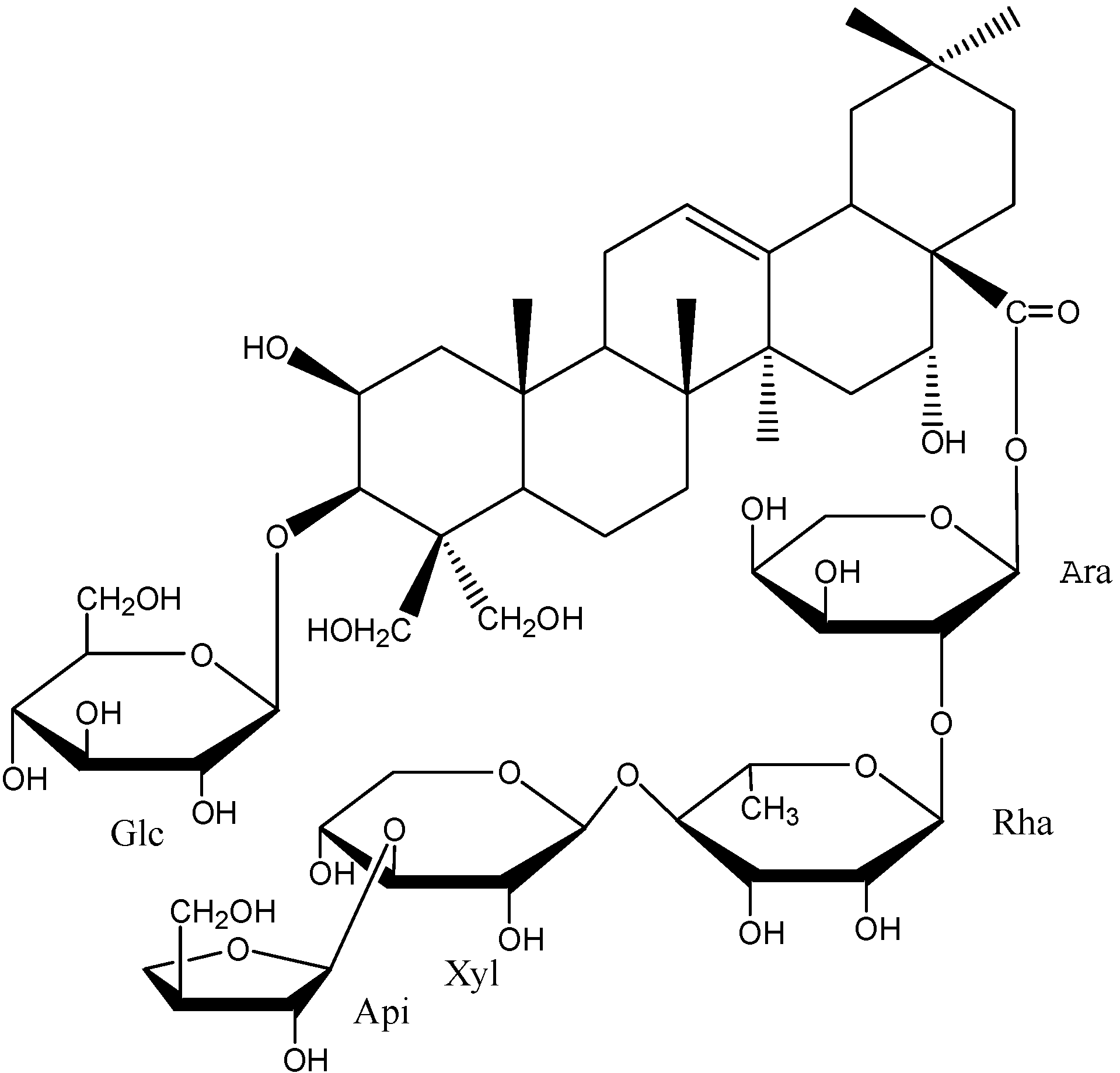
2. Results and Discussion
2.1. Effects of Different Concentrations of PD on the Proliferation of Human Glioma U251 Cells

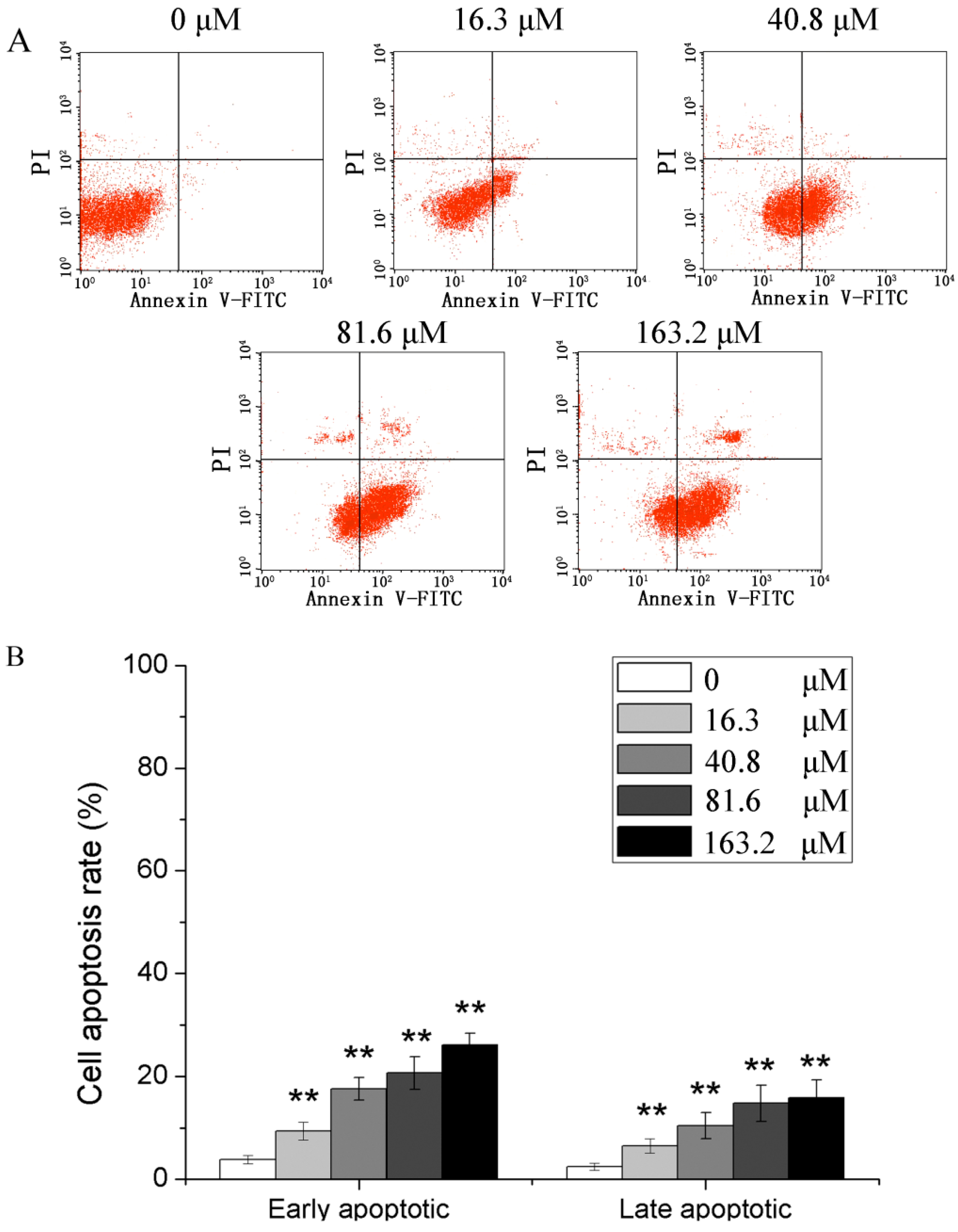
2.2. Effects of Different Concentrations of PD on the Apoptotic Rate of Human Glioma U251 Cells
2.3. Effects of Different Concentrations of PD on the Apoptotic Index Human Glioma U251 Cells

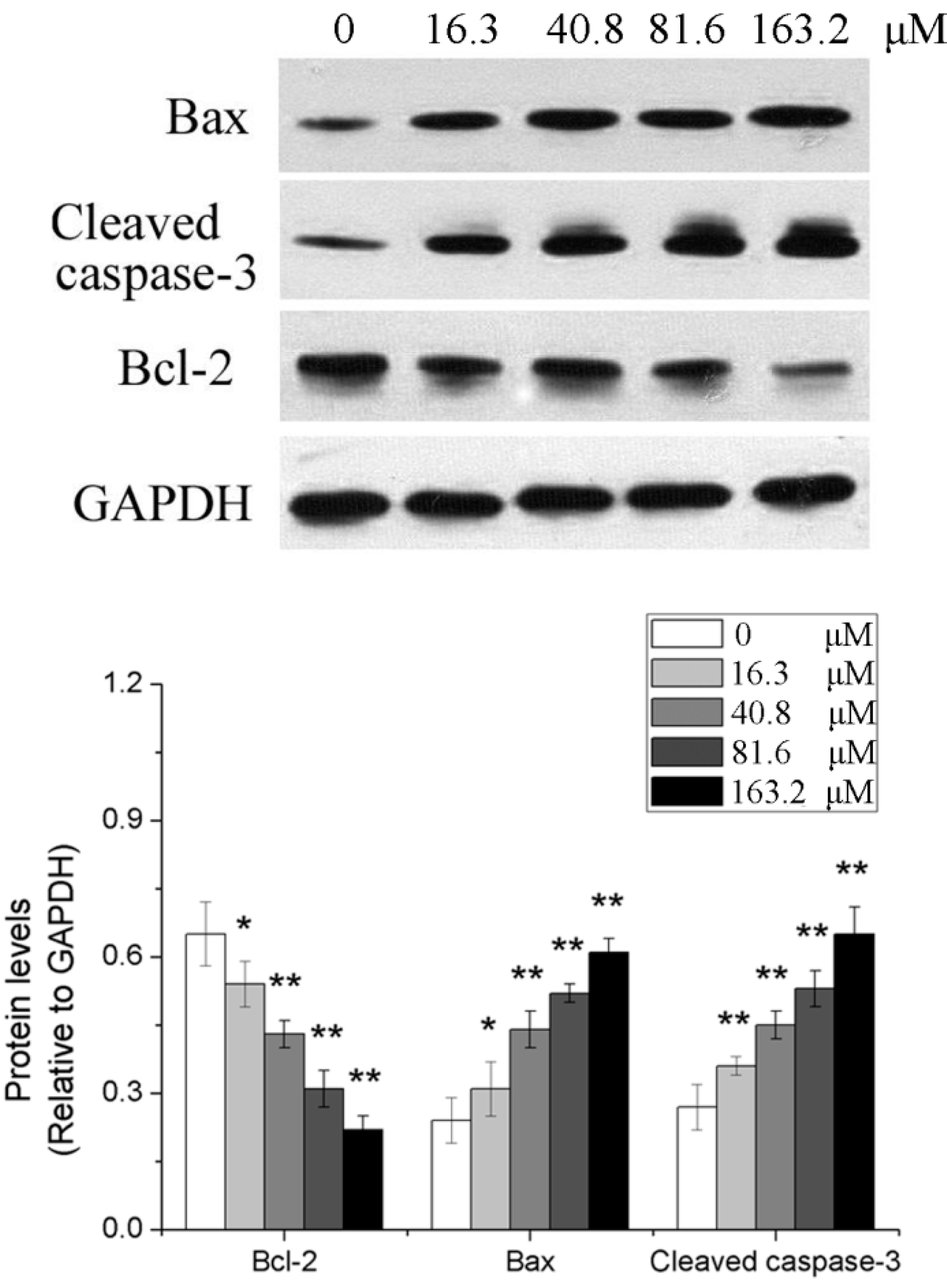
2.4. Effects of Different Concentrations of PD on the Expression of Apoptosis-Related Genes in Human Glioma U251 Cells
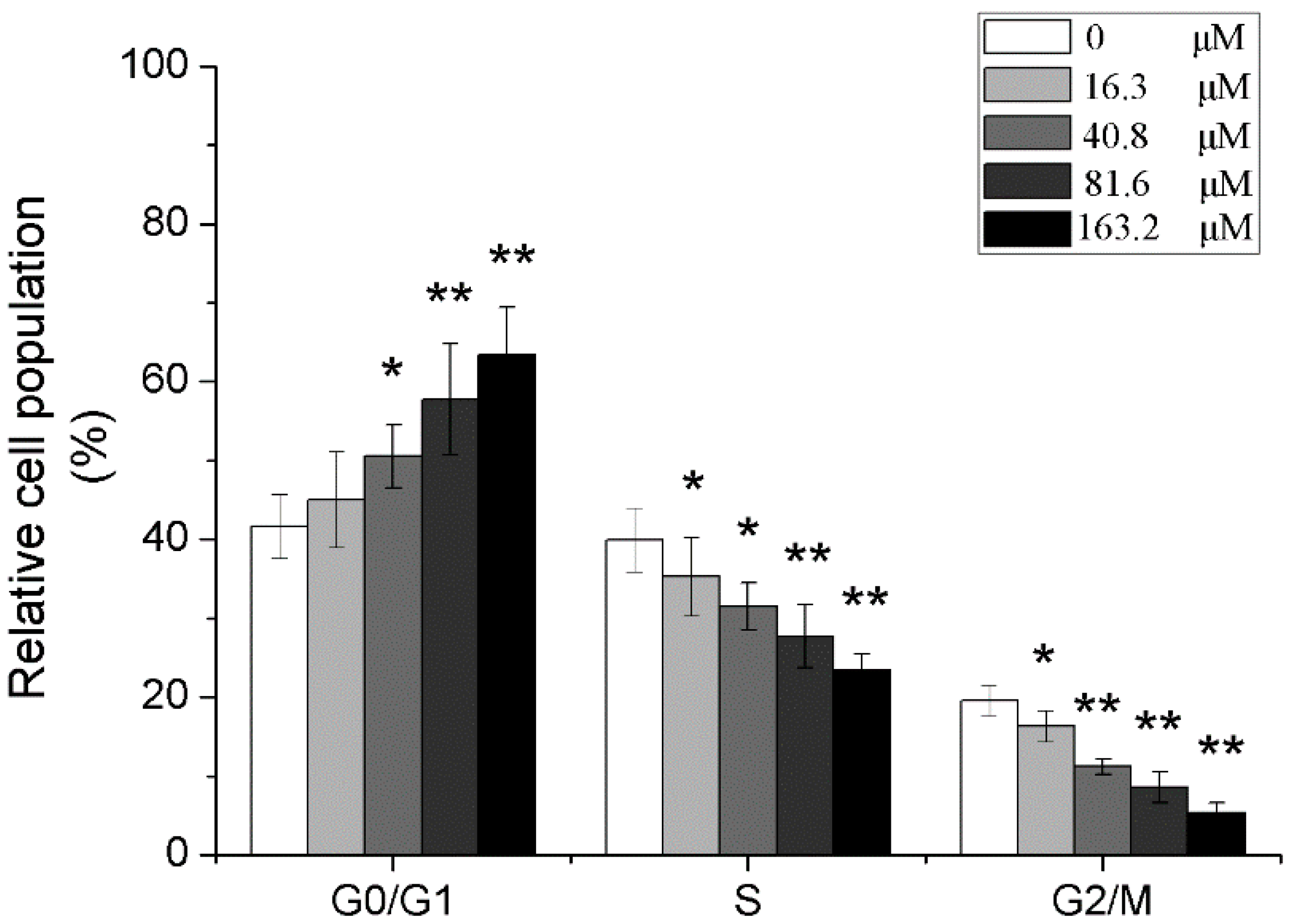
2.5. Effects of Different Concentrations of PD on the Cell Cycle of Human Glioma U251 Cells
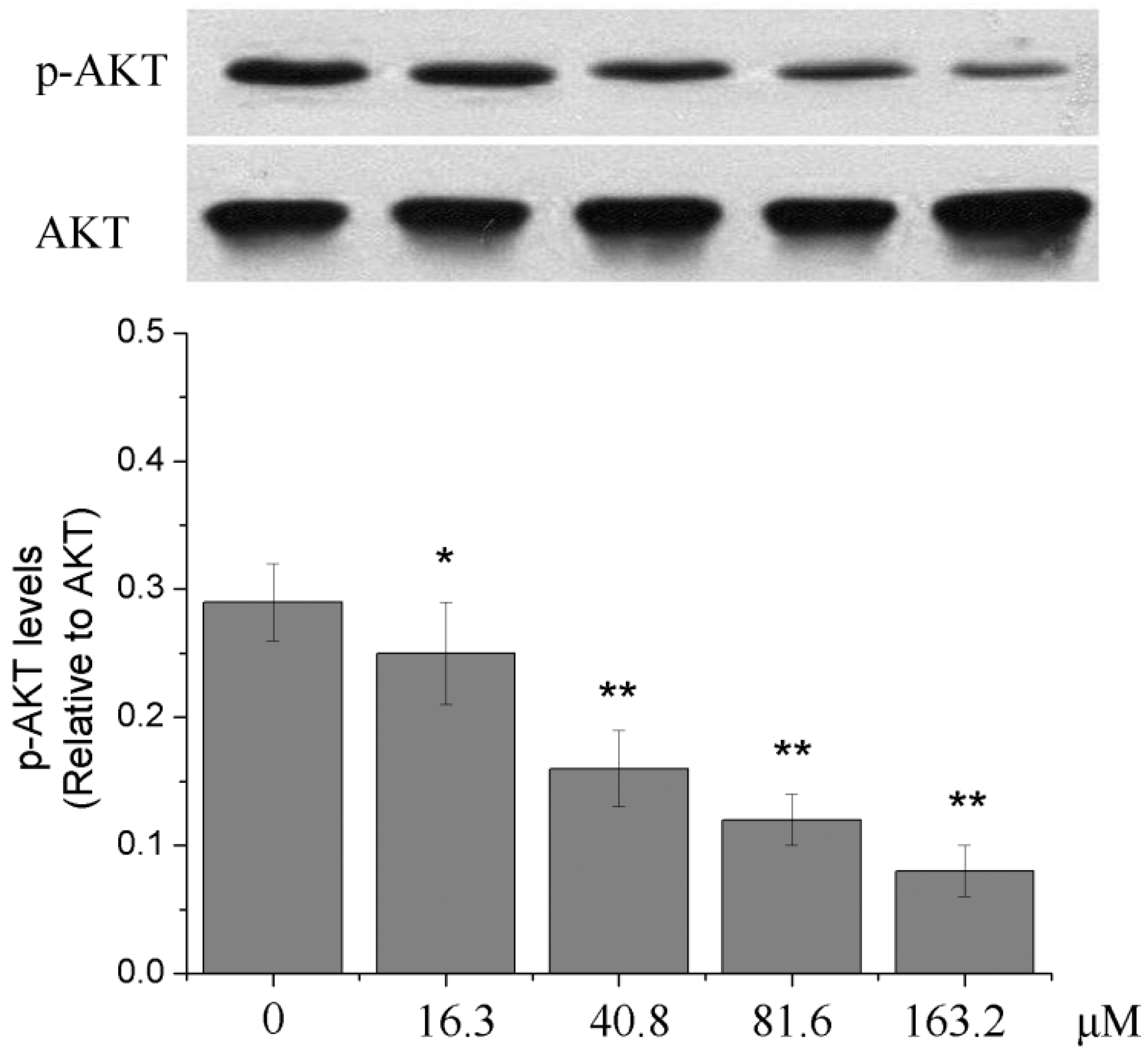
2.6. Effects of Different Concentrations of PD on the PI3K/Akt Signaling Pathway of Human Glioma U251 Cells
2.7. Discussion
3. Experimental Section
3.1. Materials and Chemicals
3.2. Detection of Cell Proliferation Activity
3.3. Detection of Cell Apoptosis and Cell Cycle
3.4. Detection of Apoptotic Index
3.5. Western Blotting Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jovčevska, I.; Kočevar, N. Glioma and glioblastoma-how much do we (not) know? Mol. Clin. Oncol. 2013, 1, 935–941. [Google Scholar] [PubMed]
- Gurney, J.G.; Kadan-Lottick, N. Brain and other central nervous system tumors: Rates, trends, and epidemiology. Curr. Opin. Oncol. 2001, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, M.; Minn, Y. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro Oncol. 2002, 4, 278–299. [Google Scholar] [PubMed]
- Partap, S.; Fisher, P.G. Update on new treatments and developments in childhood brain tumors. Curr. Opin. Pediatr. 2007, 19, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, M.P.; Semerdjieva, M.L. Postoperative chemo-radiotherapy with temodal in patients with glioblastoma multiforme-survival rates and prognostic factors. Folia Med. (Plovdiv) 2010, 52, 26–33. [Google Scholar]
- Fernández, A.; Sessel, S. Selective antagonism of anticancer drugs for side-effect removal. Trends Pharmacol. Sci. 2009, 30, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, V.; Palladini, G. Bortezomib-induced paralytic ileus is a potential gastrointestinal side effect of this first-in-class anticancer proteasome inhibitor. Eur. J. Gastroenterol. Hepatol. 2007, 19, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Kim, Y.J. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Cho, C.H. A novel anticancer effect of Astragalus saponins: Transcriptional activation of NSAID-activated gene. Int. J. Cancer 2009, 125, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Yonemori, K. Phase I and pharmacokinetic study of nab-paclitaxel, nanoparticle albumin-bound paclitaxel, administered weekly to Japanese patients with solid tumors and metastatic breast cancer. Cancer Chemother. Pharmacol. 2012, 69, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Minelli, R.; Cavalli, R. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur. J. Pharm. Sci. 2012, 47, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, E.; Jeong, J.H. Platycosides from the Roots of Platycodon grandiflorum and Their Health Benefits. Prev. Nutr. Food Sci. 2014, 19, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, H.A. Platycodon grandiflorus alleviates DNCB-induced atopy-like dermatitis in NC/Nga mice. Indian J. Pharmacol. 2012, 44, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Fu, J.N. Platycoside O, a new triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2011, 16, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W. Platycoside N: A new oleanane-type triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2010, 15, 8702–8708. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yoo, D.S. Platyconic acid A, a genuine triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2008, 13, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Kim, H.K. Anti-inflammatory effects of saponins derived from the roots of Platycodon grandiflorus in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Med. 2013, 31, 1357–1366. [Google Scholar] [PubMed]
- Kim, J.W.; Park, S.J. Triterpenoid Saponins Isolated from Platycodon grandiflorum Inhibit Hepatitis C Virus Replication. Evid. Based Complement. Altern. Med. 2013, 2013, 560417. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, T.W. Protective effect of the roots extract of Platycodon grandiflorum on bile duct ligation-induced hepatic fibrosis in rats. Hum. Exp. Toxicol. 2013, 32, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Pan, H. A promising balanced Th1 and Th2 directing immunological adjuvant, saponins from the root of Platycodon grandiflorum. Vaccine 2008, 26, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, E.K. Crude saponins from Platycodon grandiflorum induce apoptotic cell death in RC-58T/h/SA#4 prostate cancer cells through the activation of caspase cascades and apoptosis-inducing factor. Oncol. Rep. 2013, 29, 1421–1428. [Google Scholar] [PubMed]
- Qin, H.; Du, X. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014, 35, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Pan, R. Platycodon grandiflorum induces apoptosis in SKOV3 human ovarian cancer cells through mitochondrial-dependent pathway. Am. J. Chin. Med. 2010, 38, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Kim, A.K. Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J. Med. Food 2010, 13, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Joo, E.J. Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J. Cell. Biochem. 2013, 114, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Moon, D.O. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008, 261, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Kaneiwa, Y. Studies on the saponins of the root of Platycodon grandiflorum A. De Candolle. I. Isolation and the structure of platycodin-D. Chem. Pharm. Bull. (Tokyo) 1975, 23, 2965–2972. [Google Scholar] [CrossRef]
- Feng, X.; Li, L. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: Involvement of apoptosis and autophagy. Biochem. Biophys. Res. Commun. 2014, 444, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Zar, P.P.; Yano, S. In vitro anticancer activity of loquat tea by inducing apoptosis in human leukemia cells. Biosci. Biotechnol. Biochem. 2014, 13, 1–7. [Google Scholar]
- Lim, J.H.; Lee, Y.M. Anticancer activity of hispidin via reactive oxygen species-mediated apoptosis in colon cancer cells. Anticancer Res. 2014, 34, 4087–4093. [Google Scholar] [PubMed]
- Wimardhani, Y.S.; Suniarti, D.F. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ma, Z.Y. Thiosemicarbazone Cu (II) and Zn (II) complexes as potential anticancer agents: Syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 2014, 136, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Vallette, F. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [PubMed]
- Su, J.; Zhou, L. Bcl-2 Family Proteins Are Involved in the Signal Crosstalk between Endoplasmic Reticulum Stress and Mitochondrial Dysfunction in Tumor Chemotherapy Resistance. Biomed. Res. Int. 2014, 2014, 234370. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Teijido, O. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x (L): Keep your friends close but your enemies closer. Int. J. Biochem. Cell Biol. 2013, 45, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl-2 family: Regulators of the cellular life or death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Penthala, N.R.; Bommagani, S. Heck products of parthenolide and melampomagnolide-B as anticancer modulators that modify cell cycle progression. Eur. J. Med. Chem. 2014, 85, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Boerries, M. The natural anticancer compound rocaglamide selectively inhibits the G1-S-phase transition in cancer cells through the ATM/ATR-mediated Chk1/2 cell cycle checkpoints. Int. J. Cancer 2014, 134, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Pandeti, S.; Sharma, K. Synthesis of novel anticancer iridoid derivatives and their cell cycle arrest and caspase dependent apoptosis. Phytomedicine 2014, 21, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Ammirante, M. Apoptosis inhibition in cancer cells: A novel molecular pathway that involves BAG3 protein. Int. J. Biochem. Cell Biol. 2007, 39, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Gao, L. A genome-wide RNAi screen identifies FOXO4 as a metastasis-suppressor through counteracting PI3K/AKT signal pathway in prostate cancer. PLoS One 2014, 9, e101411. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Duan, L. Activation of the PI3K/Akt/mTOR/p70S6K pathway is involved in S100A4-induced viability and migration in colorectal cancer cells. Int. J. Med. Sci. 2014, 11, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.L.; Jung, K.H. Anticancer activity of HS-527, a novel inhibitor targeting PI3-kinase in human pancreatic cancer cells. Cancer Lett. 2014, 353, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.R. Current status and future perspectives of PI3K and mTOR inhibitor as anticancer drugs in breast cancer. Curr. Cancer Drug Targets 2013, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, J.; Sheikh, A. Akt inhibitors: Mechanism of action and implications for anticancer therapeutics. Infect. Agents Cancer 2013, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds platycodin D are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Sun, G.; Yuan, G.; Wang, R.; Sun, X. Effects of Platycodin D on Proliferation, Apoptosis and PI3K/Akt Signal Pathway of Human Glioma U251 Cells. Molecules 2014, 19, 21411-21423. https://doi.org/10.3390/molecules191221411
Xu C, Sun G, Yuan G, Wang R, Sun X. Effects of Platycodin D on Proliferation, Apoptosis and PI3K/Akt Signal Pathway of Human Glioma U251 Cells. Molecules. 2014; 19(12):21411-21423. https://doi.org/10.3390/molecules191221411
Chicago/Turabian StyleXu, Chong, Guibo Sun, Guangxin Yuan, Rui Wang, and Xiaobo Sun. 2014. "Effects of Platycodin D on Proliferation, Apoptosis and PI3K/Akt Signal Pathway of Human Glioma U251 Cells" Molecules 19, no. 12: 21411-21423. https://doi.org/10.3390/molecules191221411



