Chemical Constituents from Licania cruegeriana and Their Cardiovascular and Antiplatelet Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Isolation
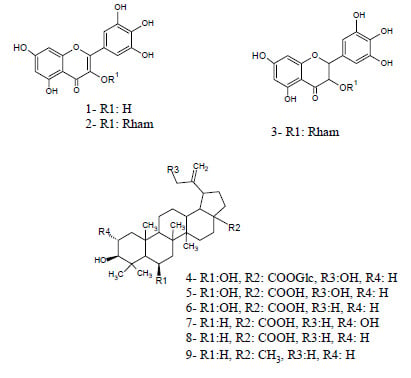
2.2. Structure Elucidation of Compounds 4, 5 and 6
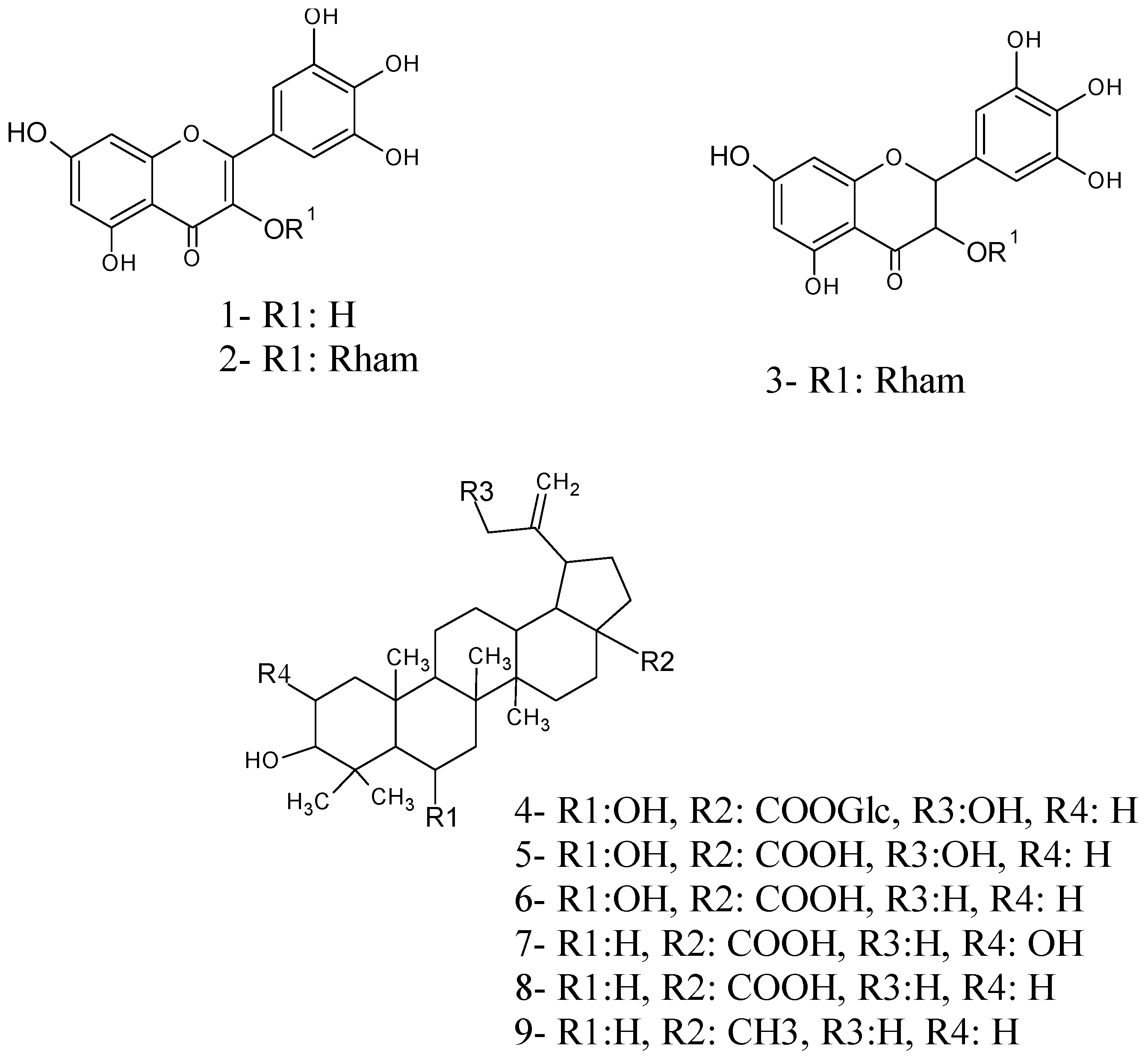
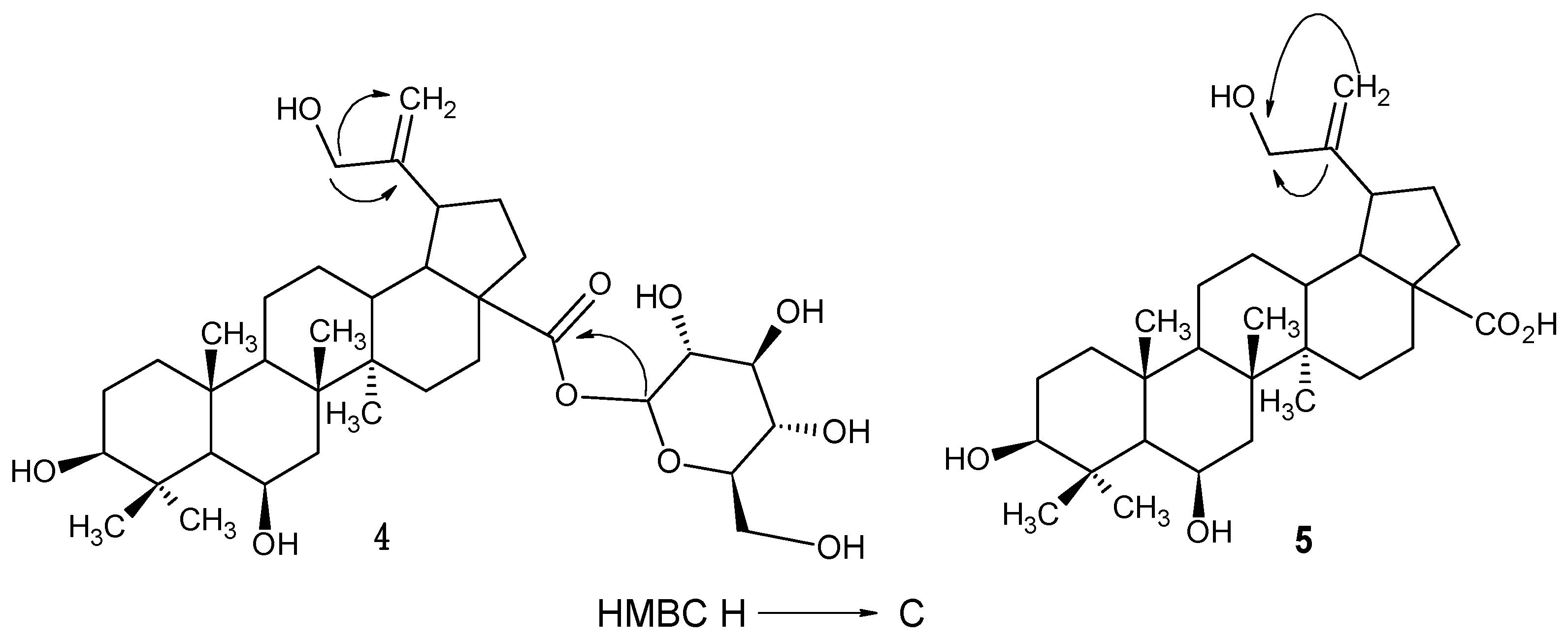
2.3. Cardiovascular Effects of the Isolated Compounds
2.4. Antiplatelet Effects of Isolated Compounds
| Compounds | MABP (mmHg) Basal | MABP (mmHg) After Treatment | Maximal (%) Change | HR (bpm) Basal | HR (bpm) after Treatment | Maximal (%) Change | Time Peak (s) | Time Recovery (min) |
|---|---|---|---|---|---|---|---|---|
| Vehicle | 141 ± 8 | 137 ± 5 | −3.1 | 422 ± 21 | 414 ± 12 | −1.8 | - | - |
| 5 | 138 ± 5 | 55 ± 10 ** | −60.1 ** | 290 ± 15 | 330 ± 12 * | 11.0 | 180 ± 20 | >45 |
| 6 | 145 ± 10 | 120 ± 12 * | −17.2 * | 405 ± 18 | 572 ± 16 ** | 41.2 | 180 ± 10 | >45 |
| Losartan | 145 ± 12 | 117 ± 9 * | −19.3 * | 350 ± 32 | 447 ± 21 ** | 27.7 | 600 ± 9 | >45 |
| Compounds | Aggregation (%) | ||
|---|---|---|---|
| AA | Collagen | ADP | |
| Control | 100 | 100 | 100 |
| 3 | 1.2 ± 0 *** | 18.7 ± 6 ** | 40.1 ± 6 * |
| 4 | 49.5 ± 6 | 13.6 ± 6 *** | 90.6 ± 5 |
| 5 | 87.5 ± 2 | 18.5 ± 7 ** | 22.9 ± 5 ** |
| 6 | 89.1 ± 4 | 32.5 ± 6 * | 61.6 ± 5 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Spectral Data
3.4. Biological Assays
3.4.1. Cardiovascular Assay
3.4.2. In Vitro Platelet Aggregation Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Toledo, C.L.; Kubitzki, K.; Prance, G.H. Flora de Venezuela; Ediciones Fundación Educación Ambiental: Caracas, Venezuela, 1982; Volume 1, pp. 326–350.
- Prance, G.T. A new species of Licania (Chrysobalanaceae) from Cordillera del Cóndor, Ecuador. Phytokeys 2013, 26, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Pittier, H. Manual de las plantas usuales de Venezuela; Editorial Fundación Eugenio Mendoza: Caracas, Venezuela, 1978; p. 579.
- Alves-Feitosa, E.; Satiro Xavier, H.; Perrelli Randau, K. Chrysobalanaceae: Traditional uses, phytochemistry and pharmacology. Rev. Bras. Farmacogn. 2012, 22, 1181–1186. [Google Scholar] [CrossRef]
- Cartaxo, S.L.; Souza, M.M.A.; Albuquerque, U.P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.R.; Peres, A.; Gallori, S.; Vincieri, F. Determination of myricetin derivatives in Chrysobalanus icaco L. (Chrysobalanaceae). Rev. Bras. Farmacogn. 2006, 16, 333–337. [Google Scholar] [CrossRef]
- Braca, A.; Bilia, A.; Mendez, J.; Morelli, I. Myricetin glycosides from Licania densiflora. Fitoterapia 2001, 72, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Sholichin, M.; Yamasaki, K.; Kasai, R.; Tanaka, O. 13C Nuclear magnetic resonance of lupane-type triterpenes, lupeol, betulin and betulinic acid. Chem. Pharm. Bull. 1980, 28, 1006–1008. [Google Scholar] [CrossRef]
- Lunardi, I.; Peixoto, J.; da Silva, C.; Shuquel, I.; Basso, E.; Vidotti, G. Triterpenic acids from Eugenia moraviana. J. Braz. Chem. Soc. 2001, 12, 180–183. [Google Scholar] [CrossRef]
- Cerri, R.; Aquino, R.; de Simone, F.; Pizza, C. New quinovic acid glycosides from Uncaria tomentosa. J. Nat. Prod. 1988, 51, 257–261. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Xuan, L. Two new lupene-type triterpenoids from the roots of Liquidambar formosana. Nat. Prod. Res. 2012, 26, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, C.; Liu, Y.; Xu, Q.; Li, X.; Yang, S. New triterpenoids and other constituents from the fruits of Benincasa hispida (Thunb.) cogn. J. Agric. Food Chem. 2013, 61, 12692–12699. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Jan, S.; Abbaskhan, A.; Musharraf, S.; Samina, S. Atta-ur-Rahman. Cycloartane triterpenoids from Astragalus bicuspis. J. Nat. Prod. 2008, 71, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Tomita, Y.; Tori, K.; Yoshimura, Y. Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1978, 100, 3331–3339. [Google Scholar] [CrossRef]
- Kuang, H.; Li, H.; Wang, Q.; Yang, B.Y.; Wang, Z.; Xia, Y. Triterpenoids from the roots of Sanguisorba tenuifolia var. Alba. Molecules 2011, 16, 4642–4651. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, X.; Hu, Y.; Liu, Y.; Liu, K.; Zhang, F.; Zhou, X. Two new triterpenoids from Lysimachia heterogenea klatt and evaluation of their cytotoxicity. Molecules 2011, 16, 8076–8082. [Google Scholar] [CrossRef] [PubMed]
- Prakash Chaturvedula, V.; Chen, S.; Yu, O.; Mao, G. Isolation, NMR spectral analysis and hydrolysis studies of a hepta pyranosyl diterpene glycoside from Stevia rebaudiana Bertoni. Biomolecules 2013, 3, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Collister, J.P.; Hornfeldt, B.J.; Osborn, J.W. Hypotensive response to losartan in normal rats role of ang II and the area postrema. Hypertension 1996, 27, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Bądzyńska, B.; Lipkowski, A.W.; Sadowski, J.; Kompanowska-Jezierska, E. Vascular effects of a tripeptide fragment of novokinine in hypertensive rats: Mechanism of the hypotensive action. Pharmacol. Rep. 2014, 66, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, E.; Blanc, J.; Elghozi, J.L. Effects of losartan on short-term variability of blood pressure in SHR and WKY rats. Fundam. Clin. Pharmacol. 1995, 9, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Castillo, C.; Estrada, O.; Carvajal, E. Pomolic acid triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets. Phytomedicine 2012, 19, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Morelli, I.; Mendez, J.; Battinelli, L.; Braghiroli, L.; Mazzanti, G. Antimicrobial triterpenoids from Licania heteromorpha. Planta Med. 2000, 8, 768–769. [Google Scholar] [CrossRef]
- Estrada, O.; Alvarado-Castillo, C.; Fernández, A.; López, M.; Romero-Vecchione, E.; Vásquez, J.; Méndez, J.; Conde, D.; Cardozo, A. Pomolic Acid Isolated from the Leaves of Licania pittieri Inhibits ADP-and Epinephrine-Induced Platelet Aggregation and has Hypotensive Effect on Rats. Curr. Bioact. Compd. 2009, 5, 219–225. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.J.; Navarrete-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, O.; Contreras, W.; Acha, G.; Lucena, E.; Venturini, W.; Cardozo, A.; Alvarado-Castillo, C. Chemical Constituents from Licania cruegeriana and Their Cardiovascular and Antiplatelet Effects. Molecules 2014, 19, 21215-21225. https://doi.org/10.3390/molecules191221215
Estrada O, Contreras W, Acha G, Lucena E, Venturini W, Cardozo A, Alvarado-Castillo C. Chemical Constituents from Licania cruegeriana and Their Cardiovascular and Antiplatelet Effects. Molecules. 2014; 19(12):21215-21225. https://doi.org/10.3390/molecules191221215
Chicago/Turabian StyleEstrada, Omar, Whendy Contreras, Giovana Acha, Eva Lucena, Whitney Venturini, Alfonso Cardozo, and Claudia Alvarado-Castillo. 2014. "Chemical Constituents from Licania cruegeriana and Their Cardiovascular and Antiplatelet Effects" Molecules 19, no. 12: 21215-21225. https://doi.org/10.3390/molecules191221215