Four New Furostanol Saponins from the Rhizomes and Roots of Smilax scobinicaulis and Their Cytotoxicity
Abstract
:1. Introduction

2. Results and Discussion
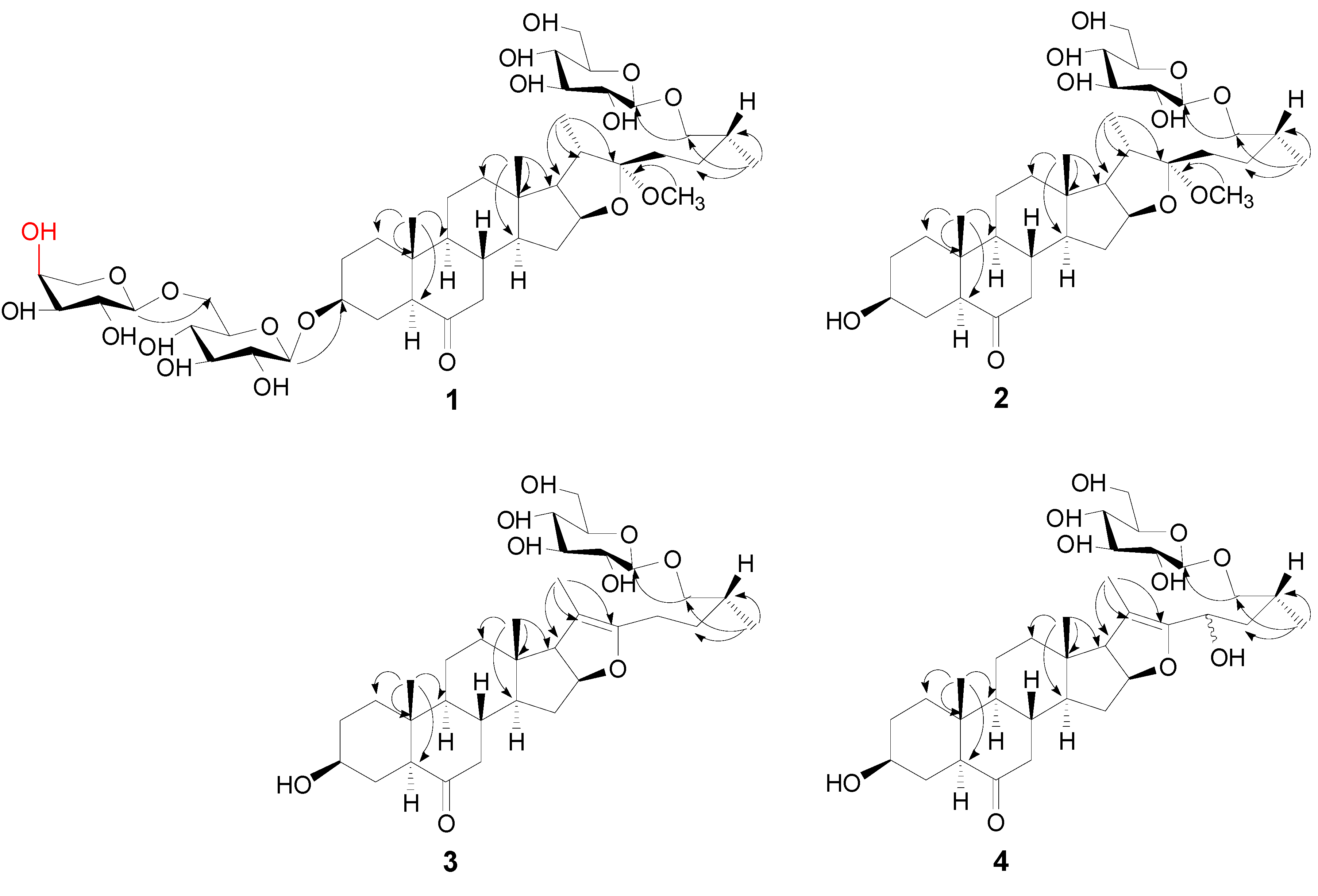
2.1. Structure Elucidation
2.2. Cytotoxic Activity
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. New Compound Data
| NO. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH a | δC b | δH a | δC b | δH a | δC b | δH c | δC d | |
| 1 | 1.80 (m) 1.35 (m) | 36.3 | 1.79 (m) 1.33 (m) | 36.3 | 1.80 (m) 1.32 (m) | 36.3 | 1.64 (m) 1.16 (m) | 36.8 |
| 2 | 1.93 (m) 1.32 (m) | 28.6 | 1.82 (m) 1.64 (m) | 30.1 | 1.42 (m) 1.31 (m) | 30.1 | 1.65 (m) 2.04 (m) | 31.6 |
| 3 | 3.36 (m) | 76.8 | 3.53 (m) | 69.7 | 3.52 (m) | 69.7 | 3.83 (m) | 69.8 |
| 4 | 1.43 (m) 2.03 (m) | 26.0 | 1.41 (m) 1.81 (m) | 29.3 | 1.43 (m) 1.83 (m) | 29.3 | 1.90 (m) 2.29 (m) | 31.0 |
| 5 | 2.40 (d, 12.0) | 56.0 | 2.38 (d, 11.0) | 56.2 | 2.37 (dd, 12.5, 2.0) | 56.2 | 2.26 (m) | 56.8 |
| 6 | – | 212.0 | – | 212.0 | – | 212.0 | – | 209.7 |
| 7 | 2.16 (t, 13.0) 2.24 (m) | 46.1 | 2.14 (t, 12.5) 2.25 (dd, 13.0, 4.5) | 46.1 | 2.15 (m) 2.28 (dd, 13.0, 4.5) | 46.2 | 2.02 (m) 2.35 (m) | 46.8 |
| 8 | 2.00 (m) | 37.4 | 2.00 (m) | 37.4 | 1.97 (m) | 37.3 | 1.81 (m) | 37.0 |
| 9 | 1.40 (m) | 53.4 | 1.40 (m) | 53.4 | 1.36 (m) | 53.4 | 1.15 (m) | 53.5 |
| 10 | – | 40.8 | – | 40.6 | – | 40.6 | – | 40.7 |
| 11 | 1.42 (m) 1.70 (m) | 21.0 | 1.42 (m) 1.68 (m) | 21.0 | 1.42 m 1.72 m | 21.2 | 1.52 (m) 1.73 (m) | 21.5 |
| 12 | 1.28 (m) 1.82 (m) | 39.1 | 1.25 (m) 1.82 (m) | 39.2 | 1.37 m 1.88 m | 39.0 | 1.22 (m) | 39.2 |
| 13 | – | 41.1 | – | 41.1 | – | 43.6 | – | 43.9 |
| 14 | 1.43 (m) | 56.0 | 1.41 (m) | 56.0 | 1.45 (m) | 54.6 | 1.06 (m) | 54.6 |
| 15 | 1.30 (m) 1.95 (m) | 31.1 | 1.30 (m) 1.94 (m) | 31.2 | 1.45 (m) 2.15 (m) | 33.5 | 1.38 (m) 1.97 (m) | 33.9 |
| 16 | 4.40 (m) | 80.8 | 4.41 (q–like, 7.5) | 80.6 | 4.78 (m) | 84.0 | 4.78 (m) | 84.1 |
| 17 | 1.80 (m) | 63.6 | 1.79 (m) | 63.6 | 2.56 (d, 10.0) | 64.0 | 2.48 (d, 10.5) | 64.5 |
| 18 | 0.86 (s) | 15.5 | 0.85 (s) | 15.5 | 0.72 (s) | 13.3 | 0.66 (s) | 14.2 |
| 19 | 0.80 (s) | 12.1 | 0.79 (s) | 12.1 | 0.79 (s) | 12.0 | 0.75 (s) | 13.0 |
| 20 | 2.21 (m) | 39.8 | 2.21 (m) | 39.8 | – | 103.6 | – | 104.6 |
| 21 | 1.04 (d, 6.0) | 14.7 | 1.03 (d, 7.0) | 14.5 | 1.63 (s) | 10.5 | 1.76 (s) | 11.4 |
| 22 | – | 112.6 | – | 112.6 | – | 151.8 | 154.3 | |
| 23 | 1.63 (m) 1.84 (m) | 29.9 | 1.62 (m) 1.81 (m) | 29.9 | 2.16 (m) | 22.7 | 4.91 (t, 7.0) | 63.6 |
| 24 | 1.16 (m) 1.62 (m) | 27.6 | 1.16 (m) 1.61 (m) | 27.2 | 1.27 (m) 1.65 (m) | 30.6 | 1.74 (m) 2.38 (m) | 39.5 |
| 25 | 1.76 (m) | 33.6 | 1.75 (m) | 33.6 | 1.78 (m) | 32.7 | 2.44 (m) | 30.7 |
| 26 | 3.40 (m) 3.76 (m) | 74.6 | 3.40 (dd, 9.5, 6.5) 3.75 (dd, 9.5, 6.5) | 74.6 | 3.41 (dd, 9.5, 6.0) 3.73 (dd, 9.5, 7.0) | 74.4 | 3.79 (m) 4.03 (m) | 75.3 |
| 27 | 0.98 (d, 5.0) | 15.9 | 0.97 (d, 7.0) | 16.0 | 0.97 (d, 6.5) | 15.9 | 1.17 (d, 6.0) | 17.6 |
| OCH3 | 3.17 (s) | 46.3 | 3.17 (s) | 46.2 | – | – | – | – |
| Glc–1' | 4.42 (d, 6.5) | 100.9 | – | – | – | – | – | – |
| 2' | 3.19 (m) | 73.7 | – | – | – | – | – | – |
| 3' | 3.37 (m) | 76.6 | – | – | – | – | – | – |
| 4' | 3.30 (m) | 70.4 | – | – | – | – | – | – |
| 5' | 3.45 (m) | 75.5 | – | – | – | – | – | – |
| 6' | 3.83 (m) 4.09 (d, 11.5) | 68.2 | – | – | – | – | – | – |
| Ara–1'' | 4.34 (d, 6.0) | 103.8 | – | – | – | – | – | – |
| 2'' | 3.60 (m) | 71.0 | – | – | – | – | – | – |
| 3'' | 3.54 (m) | 72.8 | – | – | – | – | – | – |
| 4'' | 3.71 (m) | 68.0 | – | – | – | – | – | – |
| 5'' | 3.88 (m) | 65.2 | – | – | – | – | – | – |
| Glc–1''' | 4.26 (d, 7.0) | 103.2 | 4.26 (d, 8.0) | 103.2 | 4.25 (d, 8.0) | 103.1 | 4.85 (d, 8.0) | 104.8 |
| 2''' | 3.37 (m) | 73.8 | 3.20 (t, 8.0) | 73.8 | 3.21 (t, 8.5) | 73.7 | 4.03 (m) | 75.0 |
| 3''' | 3.73 (m) | 77.1 | 3.28 (m) | 76.5 | 3.27 (m) | 76.5 | 4.22 (m) | 78.4 |
| 4''' | 3.30 (m) | 70.4 | 3.29 (m) | 70.3 | 3.32 (m) | 70.3 | 4.19 (m) | 71.6 |
| 5''' | 3.29 (m) | 76.5 | 3.35 (m) | 76.7 | 3.37 (m) | 76.7 | 3.94 (m) | 78.3 |
| 6''' | 3.67 (m) 3.90 (m) | 61.5 | 3.68 (dd, 12.0, 5.5) 3.88 (dd, 12.0, 1.5) | 61.4 | 3.70 (dd, 12.0, 6.0) 3.88 (dd, 12.0, 2.0) | 61.4 | 4.36 (m) 4.54 (d, 11.0) | 62.7 |
3.5. Acid Hydrolysis and Sugar Analysis
3.6. GC Analysis of the Sugar Moieties in 1–4
3.7. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, C.L.; Gao, J.M.; Zhu, W. Steroidal saponins from the rhizomes and roots of Smilax scobinicaulis. Phytochem. Lett. 2012, 5, 49–52. [Google Scholar] [CrossRef]
- Challinor, V.L.; Parsons, P.G.; Chap, S.; White, E.F.; Blanchfield, J.T.; Lehmann, R.P.; de Voss, J.J. Steroidal saponins from the roots of Smilax sp.: Structure and bioactivity. Steroids 2012, 77, 504–511. [Google Scholar] [CrossRef]
- Liu, J.Y.; Fu, J.X.; Gao, J.M.; Qiu, M.H. A new furostanol glycoside from the rhizomes of Smilax scobinicaulis. J. Northwest Sci.-Tech. Univ. Agric. For. 2002, 30, 222–224. [Google Scholar]
- Zhang, C.L.; Feng, S.X.; Zhang, L.X.; Ren, Z.J. A new cytotoxic steroidal saponin from the rhizomes and roots of Smilax scobinicaulis. Nat. Prod. Res. 2013, 27, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.Z.; Wen, L.S.; Ma, Y.; Shao, Y.T.; Guan, H.; Zhang, C.L. Study on chemical constituents of root of Smilax scobinicaulis. Chin. Pharm. J. 2011, 46, 980–983. [Google Scholar]
- Wang, P.; Xu, J.; Wang, Q.; Feng, S.X.; Chen, T.; Zhang, C.L. Phenylpropanoids and diphenylethene compounds from roots and rhizomes of Smilax scobinicaulis. Chin. J. Chin. Mater. Med. 2013, 38, 1531–1535. [Google Scholar]
- Zhang, C.L.; Zhu, W.; Li, X.M.; Su, B.F.; Yan, X.Y. Antisepsis activity of the rhizomes of Smilax scobinicaulis. Sci. Silvae Sin. 2006, 42, 69–73. [Google Scholar] [CrossRef]
- Kubo, S.; Mimaki, Y.; Sashida, Y.; Nikaido, T.; Ohmoto, T. Steroidal saponins from the rhizomes of Smilax Sieboldii. Phytochemistry 1992, 31, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.P.; Yao, X.S.; Tezuka, Y.; Kikuchi, T. Furostanol glycosides from bulbs of Allium Chinense. Phytochemistry 1996, 41, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.H.; Do, J.C.; Son, K.H. Five new spirostanol glycosides from the subterranean parts of Smilax sieboloii. J. Nat. Prod. 1992, 55, 1129–1135. [Google Scholar] [CrossRef]
- Matsuo, Y.; Watanabe, K.; Mimaki, Y. New Steroidal Glycosides from Rhizomes of Clintonia udensis. Biosci. Biotechnol. Biochem. 2008, 72, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Guo, Z.Y.; Xue, Y.H.; Cheng, J.; Huang, N.Y.; Zhou, Y.; Cheng, F.; Zou, K. Five new furostanol saponins from the rhizomes of Tupistra chinensis. Fitoterapia 2012, 83, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, G.Y.; Huang, J.; Chen, C.J.; Ren, B.; Lu, G.; Tan, Y.; Zhang, J.X.; Li, X.; Wang, J.H. Steroidal saponins from Fritillaria pallidiflora Schrenk. Fitoterapia 2012, 83, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.D.; Borbone, N.; Iorizzi, M.; Esposito, G.; McClintock, J.B.; Zollo, F. Bioactive asterosaponins from the starfish Luidia quinaria and Psilaster cassiope. Isolation and structure characterization by two-dimensional NMR spectroscopy. J. Nat. Prod. 2003, 66, 515–519. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Feng, S.; Wang, Q.; Cao, Y.; Sun, M.; Zhang, C. Four New Furostanol Saponins from the Rhizomes and Roots of Smilax scobinicaulis and Their Cytotoxicity. Molecules 2014, 19, 20975-20987. https://doi.org/10.3390/molecules191220975
Xu J, Feng S, Wang Q, Cao Y, Sun M, Zhang C. Four New Furostanol Saponins from the Rhizomes and Roots of Smilax scobinicaulis and Their Cytotoxicity. Molecules. 2014; 19(12):20975-20987. https://doi.org/10.3390/molecules191220975
Chicago/Turabian StyleXu, Jing, Shixiu Feng, Qi Wang, Yingli Cao, Miao Sun, and Cunli Zhang. 2014. "Four New Furostanol Saponins from the Rhizomes and Roots of Smilax scobinicaulis and Their Cytotoxicity" Molecules 19, no. 12: 20975-20987. https://doi.org/10.3390/molecules191220975




