Two New Coumarins from Talaromyces flavus
Abstract
:1. Introduction
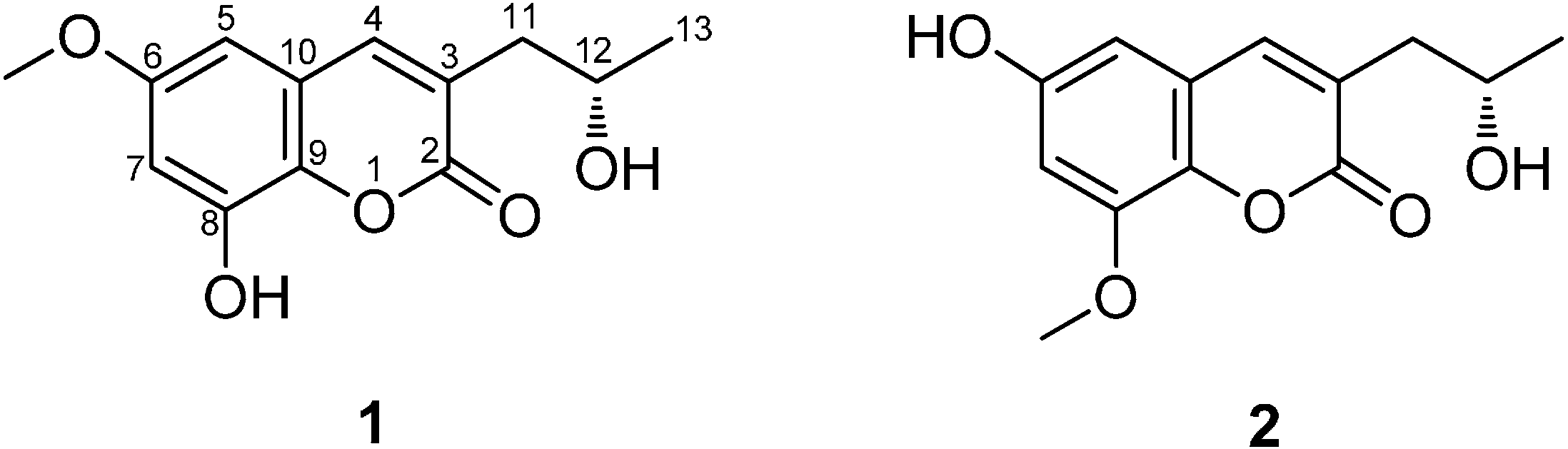
2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | ||||
| 2 | 161.0 | 160.9 | ||
| 3 | 126.5 | 126.6 | ||
| 4 | 141.1 | 7.72 (s) | 140.9 | 7.69 (s) |
| 5 | 100.1 | 6.63 (d, 2.7) | 102.8 | 6.51 (d, 2.3) |
| 6 | 155.6 | 153.9 | ||
| 7 | 104.9 | 6.61 (d, 2.7) | 102.4 | 6.66 (d, 2.3) |
| 8 | 145.1 | 147.0 | ||
| 9 | 136.4 | 135.7 | ||
| 10 | 120.2 | 119.9 | ||
| 11 | 40.3 | 2.45 (dd, 13.5, 7.8), Ha | 40.2 | 2.44 (dd, 13.6, 7.7), Ha |
| 2.54 (dd, 13.5, 4.9), Hb | 2.52 (overlapped), Hb | |||
| 12 | 64.2 | 3.92 (m) | 64.2 | 3.92 (m) |
| 13 | 23.4 | 1.10 (d, 6.2) | 23.4 | 1.09 (d, 6.1) |
| 8-OH | 10.20 (s) | |||
| 12-OH | 4.62 (d, 4.5) | |||
| 6-OCH3 | 55.4 | 3.74 (s) | ||
| 8-OCH3 | 55.9 | 3.84 (s) | ||


3. Experimental Section
3.1. General Procedures
3.2. Fungal Material and Culture
3.3. Extraction and Isolation
3.4. Spectroscopic Data
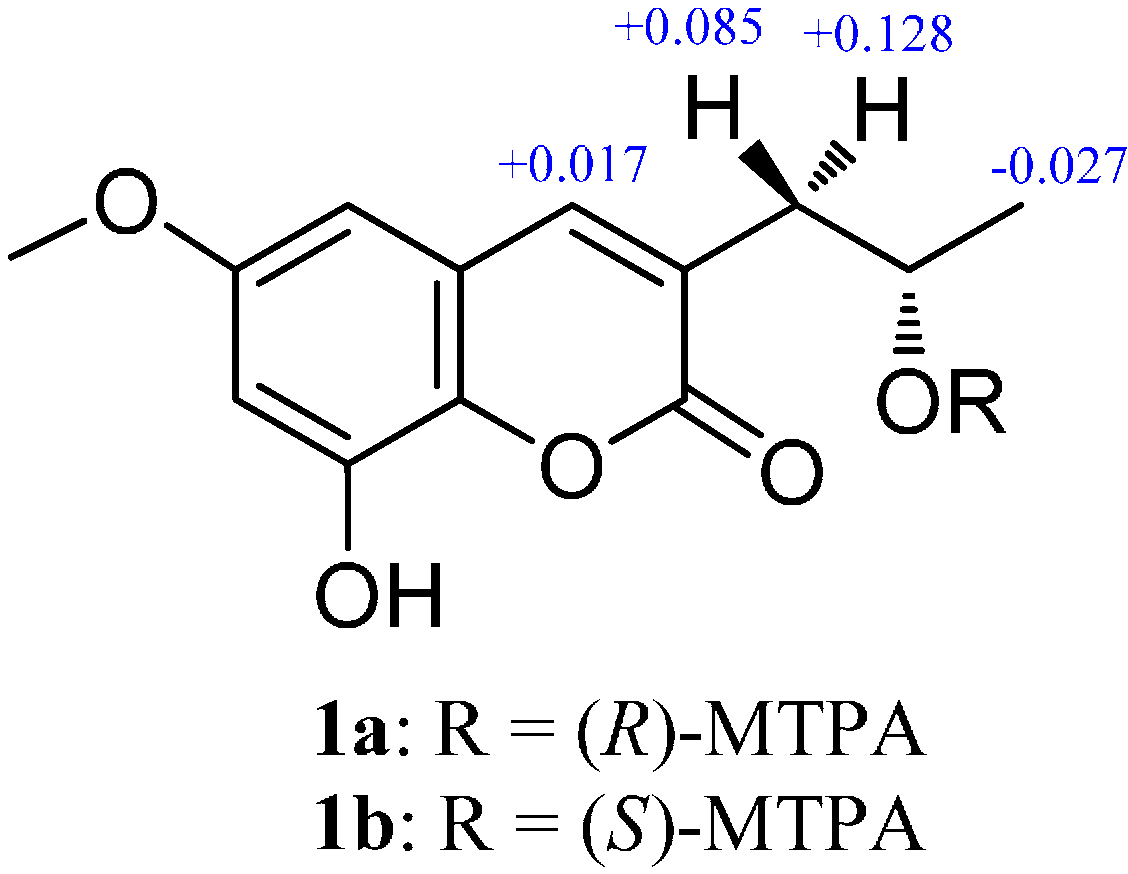
3.5. Preparation of (S)- and (R)-MTPA Esters of 1 (1a and 1b)
3.6. Absolute Configuration of the Secondary Alcohol in 2
3.7. ThT Fluorescence Assay
3.8. Cytotoxicity Assay
3.9. Antimicrobial Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bara, R.; Zerfass, I.; Aly, A.H.; Goldbach-Gecke, H.; Raghavan, V.; Sass, P.; Mándi, A.; Wray, V.; Polavarapu, P.L.; Pretsch, A.; et al. Atropisomeric dihydroanthracenones as inhibitors of multriesistant Staphylococcus aureus. J. Med. Chem. 2013, 56, 3257–3272. [Google Scholar] [CrossRef] [PubMed]
- Bara, R.; Aly, A.H.; Wray, V.; Lin, W.H.; Proksch, P.; Debbab, A. Talaromins A and B, new cyclic peptides from the endophytic fungus Talaromyces wortmannii. Tetrahedron Lett. 2013, 54, 1686–1689. [Google Scholar] [CrossRef]
- Guo, J.P.; Zhu, C.Y.; Zhang, C.P.; Chu, Y.S.; Wang, Y.L.; Zhang, J.X.; Wu, D.K.; Zhang, K.Q.; Niu, X.M. Thermolides, potent nematocidal PKS-NRPS hybrid metabolites from thermophilic fungus Talaromyces thermophilus. J. Am. Chem. Soc. 2012, 34, 20306–20309. [Google Scholar] [CrossRef]
- Li, H.X.; Huang, H.B.; Shao, C.L.; Huang, H.R.; Jiang, J.Y.; Zhu, X.; Liu, Y.Y.; Liu, L.; Lu, Y.J.; Li, M.F.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Proksa, B. Talaromyces flavus and its metabolites. Chem. Pap. 2010, 64, 696–714. [Google Scholar] [CrossRef]
- He, J.W.; Mu, Z.Q.; Gao, H.; Chen, G.D.; Zhao, Q.; Hu, D.; Sun, J.Z.; Li, X.X.; Li, Y.; Liu, X.Z.; et al. New polyesters from Talaromyces flavus. Tetrahedron 2014, 70, 4425–4430. [Google Scholar] [CrossRef]
- He, J.W.; Liang, H.X.; Gao, H.; Kuang, R.Q.; Chen, G.D.; Hu, D.; Wang, C.X.; Liu, X.Z.; Li, Y.; Yao, X.S. Talaflavuterpenoid A, a new nardosinane-type sesquiterpene from Talaromyces flavus. J. Asian Nat. Prod. Res. 2014, 16, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.S.; Edrada, R.; Müller, W.E.G.; Bayer, M.; Lin, W.H.; Wu, J.; et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Hashtmoto, M.; Hirat, T.; Takeda, I.; Sasamura, Y.; Sakamura, S.; Sato, R.; Tajimi, A. Structure, synthesis, and stereochemistry of (+)-orthosporin, a phytotoxic metabolite of Rhynchosporium orthosorum. Chem. Lett. 1989, 18, 1495–1498. [Google Scholar] [CrossRef]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and alpha-methoxy-alpha-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar]
- Gerards, M.; Snatzke, G. Circular dichroism, XCIII determination of the absolute configuration of alcohols, olefins, epoxides, and ethers from the CD of their “in situ” complexes with [Rh2(O2CCF3)4]. Tetrahedron: Asymmetry 1990, 1, 221–236. [Google Scholar] [CrossRef]
- Frelek, J.; Szczepek, W.J. [Rh2(OCOCF3)4] as an auxiliary chromophore in chiroptical studies on steroidal alcohols. Tetrahedron: Asymmetry 1999, 10, 1507–1520. [Google Scholar] [CrossRef]
- Ayer, W.A.; Racok, J.S. The metabolites of Talaromyces flavus: Part 1. Metabolites of the organic extracts. Can. J. Chem. 1990, 68, 2085–2094. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B.; Kemp, K. Nover sesquiterpenoid matrix metalloproterinase-3 inhibitors from an acid mine waste extremophile. J. Nat. Prod. 2004, 67, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Teles, H.L.; Sordi, R.; Silva, G.H.; Castro-Gamboa, I.; Bolzani, V.D.S.; Pfenning, L.H.; Abreu, L.M.D.; Costa-Neto, C.M.; Young, M.C.M.; Araújo, Ȃ.R. Aromatic compounds produced by Periconia atropurpurea, an endophytic fungus associated with Xylopia aromatica. Phytochemistry 2006, 67, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.C.; Chen, G.D.; Kong, M.Z.; Li, G.Q.; Cui, J.Y.; Li, X.X.; Wu, Z.Y.; Guo, L.D.; Cen, Y.Z.; Zheng, Y.Z.; et al. Nodulisporisteriods A and B, the first 3,4-seco-4-methyl-progesteroids from Nodulisporium sp. Steroids 2013, 78, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.-W.; Qin, D.-P.; Gao, H.; Kuang, R.-Q.; Yu, Y.; Liu, X.-Z.; Yao, X.-S. Two New Coumarins from Talaromyces flavus. Molecules 2014, 19, 20880-20887. https://doi.org/10.3390/molecules191220880
He J-W, Qin D-P, Gao H, Kuang R-Q, Yu Y, Liu X-Z, Yao X-S. Two New Coumarins from Talaromyces flavus. Molecules. 2014; 19(12):20880-20887. https://doi.org/10.3390/molecules191220880
Chicago/Turabian StyleHe, Jun-Wei, Da-Peng Qin, Hao Gao, Run-Qiao Kuang, Yang Yu, Xing-Zhong Liu, and Xin-Sheng Yao. 2014. "Two New Coumarins from Talaromyces flavus" Molecules 19, no. 12: 20880-20887. https://doi.org/10.3390/molecules191220880




