Aggregation Behavior of Long-Chain Piperidinium Ionic Liquids in Ethylammonium Nitrate
Abstract
:1. Introduction
2. Results and Discussion
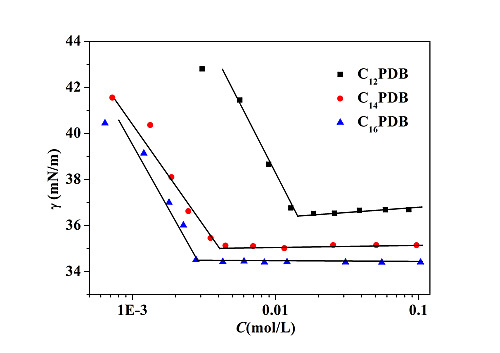
2.1. Surface Tension of CnPDB in EAN
| ILs | cmc (×103 mol/L) | γcmc (mN/m) | Πcmc (mN/m) | Гmax (μmol/m2) | Amin (Å2) |
|---|---|---|---|---|---|
| C12PDB | 13.7 ± 0.5 | 36.412 ± 0.001 | 12.935 ± 0.001 | 0.372 | 446.5 |
| C14PDB | 4.7 ± 0.03 | 35.112 ± 0.001 | 14.351 ± 0.001 | 1.201 | 138.3 |
| C16PDB | 2.8 ± 0.03 | 34.610 ± 0.001 | 14.737 ± 0.001 | 1.390 | 119.4 |

2.2. Temperature Dependence of cmc
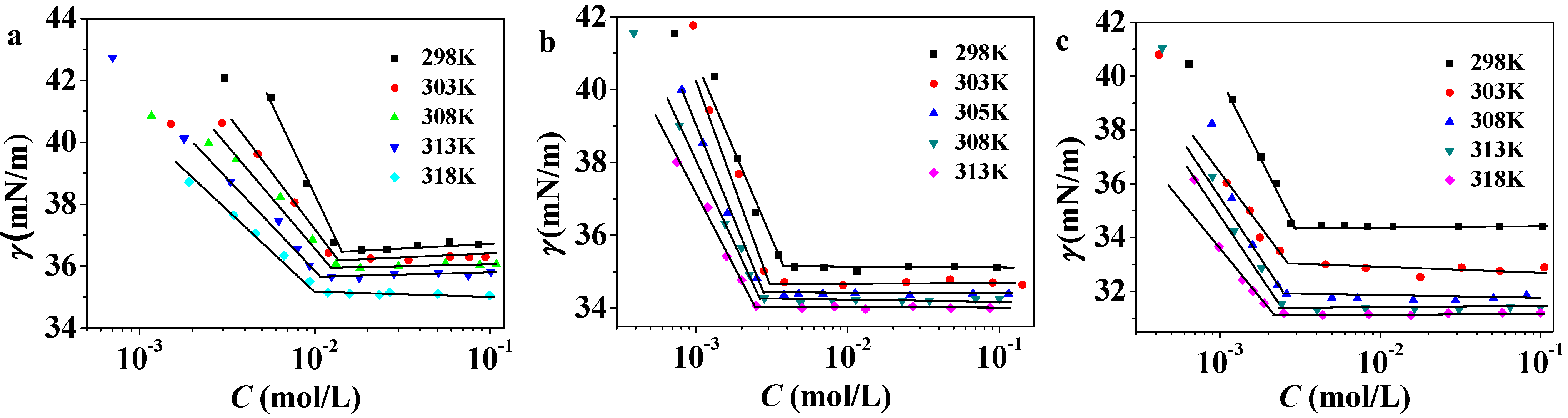
| ILs | T (K) | cmc (×103 mol·L) | (kJ·mol−1) | (kJ·mol−1) | (kJ·mol−1) |
|---|---|---|---|---|---|
| C12PDB | 298 | 13.7 ± 0.05 | −21.77 ± 0.009 | 10.73 ± 0.161 | −32.50 ± 0.152 |
| 303 | 12.5 ± 0.06 | −22.30 ± 0.012 | 9.172 ± 0.106 | −31.47 ± 0.094 | |
| 308 | 12.0 ± 0.03 | −22.83 ± 0.006 | 7.667 ± 0.053 | −30.49 ± 0.046 | |
| 313 | 11.9 ± 0.02 | −23.28 ± 0.004 | 6.210 ± 0.001 | −29.49 ± 0.003 | |
| 318 | 11.7 ± 0.04 | −23.76 ± 0.009 | 4.799 ± 0.049 | −28.56 ± 0.058 | |
| C14PDB | 298 | 4.7 ± 0.03 | −24.49 ± 0.017 | 52.94 ± 0.590 | −77.43 ± 0.607 |
| 303 | 3.5 ± 0.02 | −25.89 ± 0.016 | 36.47 ± 0.296 | −62.36 ± 0.312 | |
| 308 | 2.9 ± 0.02 | −26.48 ± 0.018 | 20.54 ± 0.011 | −47.02 ± 0.028 | |
| 313 | 2.7 ± 0.04 | −27.24 ± 0.037 | 5.109 ± 0.265 | −32.35 ± 0.228 | |
| 318 | 2.6 ± 0.01 | −27.68 ± 0.010 | −9.83 ± 0.532 | −17.85 ± 0.522 | |
| C16PDB | 298 | 2.8 ± 0.03 | −25.66 ± 0.027 | 15.47 ± 0.970 | −41.13 ± 0.943 |
| 303 | 2.6 ± 0.02 | −26.36 ± 0.020 | 12.63 ± 0.417 | −38.99 ± 0.397 | |
| 308 | 2.5 ± 0.01 | −26.99 ± 0.010 | 9.887 ± 0.119 | −36.87 ± 0.129 | |
| 313 | 2.3 ± 0.02 | −27.49 ± 0.023 | 7.231 ± 0.637 | −34.72 ± 0.660 | |
| 318 | 2.2 ± 0.02 | −28.09 ± 0.035 | 4.658 ± 0.139 | −32.75 ± 0.174 |
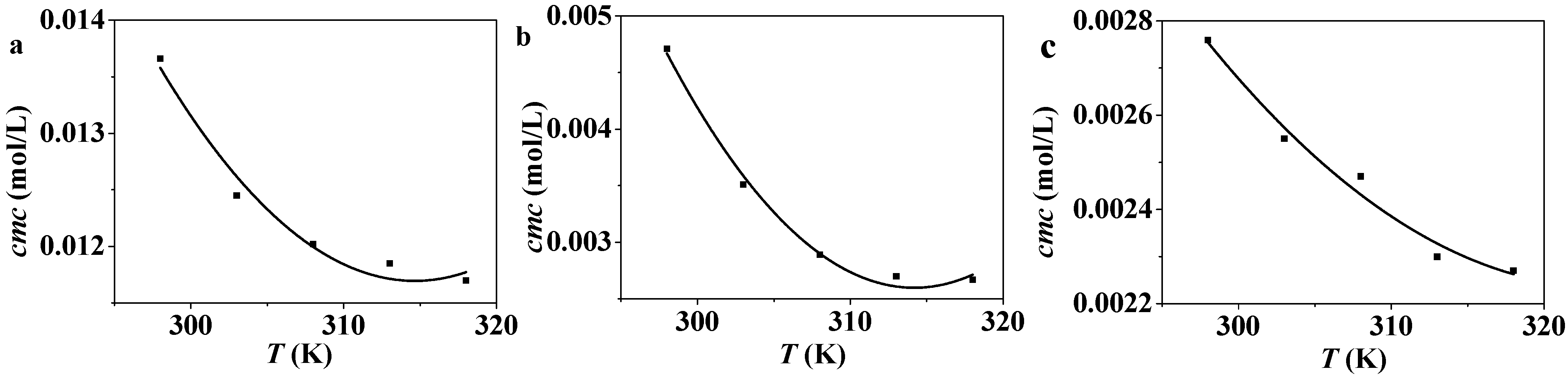
2.3. Thermodynamic Analysis on the Micelle Formation of CnPDB in EAN
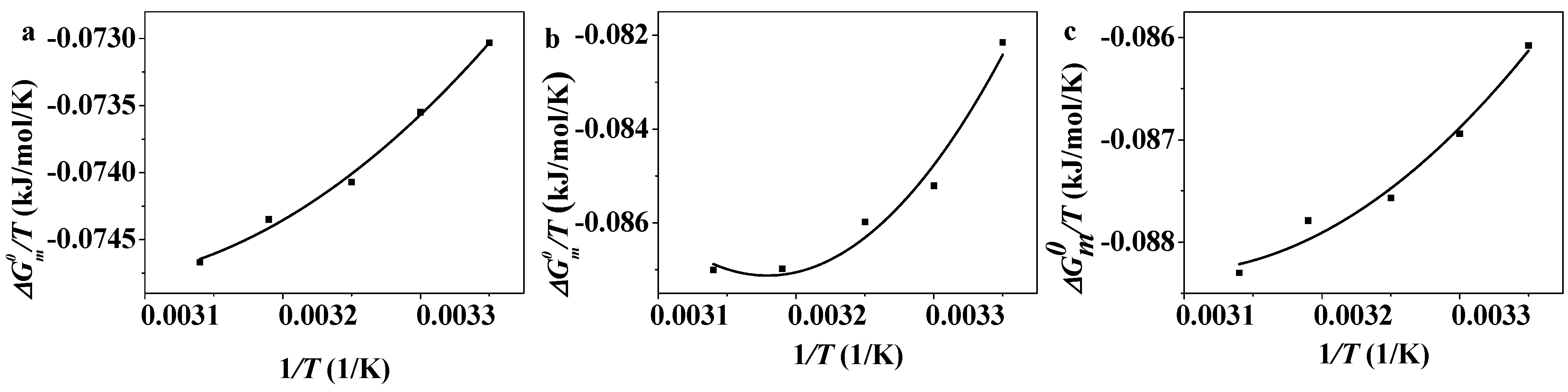

2.4. Dissipative Particle Dynamics (DPD) Simulation on the Micelle Formation of CnPDB in EAN


3. Experimental Section
3.1. Materials
3.1.1. Synthesis of CnPDB (n = 12, 14, 16)
3.1.2. Synthesis of EAN
3.2. Apparatus and Procedures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic liquids-new solutions for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Wasserscheid, P. Chemistry: Volatile times for ionic liquids. Nature 2006, 439, 797–797. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; de Souza, R.F.; Suarez, P.A. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, K.A.; Pandey, S. Surfactant aggregation within room-temperature ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide. Langmuir 2004, 20, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Cole-Hamilton, D.J. Homogeneous catalysis—New approaches to catalyst separation, recovery, and recycling. Science 2003, 299, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Yu, L.; Jiao, J.; Wang, R.; Sun, L. Surface adsorption and micelle formation of imidazolium-based zwitterionic surface active ionic liquids in aqueous solution. J. Colloid Interface Sci. 2013, 391, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Wang, J.; Li, Z.; Zhang, S. The first evidence for unilamellar vesicle formation of ionic liquids in aqueous solutions. Chem. Commun. 2013, 49, 5222–5224. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, Y.; Fang, L.; Yu, L.; Sun, L.; Wang, R.; Cheng, N. Electrolyte effect on the aggregation behavior of 1-butyl-3-methylimidazolium dodecylsulfate in aqueous solution. J. Colloid Interface Sci. 2013, 402, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Yu, P.; Wang, T.; Sheng, X.; Bi, Y.; Gong, Y.; Yu, L. Self-Aggregation of New Alkylcarboxylate-Based Anionic Surface Active Ionic Liquids: Experimental and Theoretical Investigations. J. Phys. Chem. B 2014, 118, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Hu, Q.; Bi, Y.; Xu, W.; Gong, Y.; Yu, L. Gels and Lyotropic Liquid Crystals: Using an Imidazolium-Based Catanionic Surfactant in Binary Solvents. Langmuir 2014, 30, 9076–9084. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future. Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Zhang, Q.; Sun, D.; Wei, X.; Zheng, L. Interaction between two homologues of cationic surface active ionic liquids and the PEO-PPO-PEO triblock copolymers in aqueous solutions. Colloid Polym. Sci. 2011, 289, 1711–1718. [Google Scholar] [CrossRef]
- Song, C.E. Enantioselective chemo-and bio-catalysis in ionic liquids. Chem. Commun. 2004, 9, 1033–1043. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H. Synthesis of CoPt nanorods in ionic liquids. J. Am. Chem. Soc. 2005, 127, 5316–5317. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Wrobel, R.J.; Milchert, E. Physical properties of pyridinium ionic liquids. J. Chem. Thermodyn. 2012, 55, 159–165. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M.; Ramjugernath, D.; Letcher, T.M.; Tumba, K. Phase equilibria and modeling of pyridinium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 15011–15017. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Zhang, Q.G.; Wu, F.G.; Li, Q.Z.; Yu, Z.W. Hydrogen bonding interactions between a representative pyridinium-based ionic liquid [BuPy] [BF4] and water/dimethyl sulfoxide. J. Phys. Chem. B 2010, 114, 8689–8700. [Google Scholar] [CrossRef] [PubMed]
- Neve, F.; Francescangeli, O.; Crispini, A.; Charmant, J. A2[MX4] copper (II) pyridinium salts. From ionic liquids to layered solids to liquid crystals. Chem. Mater. 2001, 13, 2032–2041. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, P.; Zhang, D.; Liu, C. Theoretical study for pyridinium-based ionic liquid 1-ethylpyridinium trifluoroacetate: Synthesis mechanism, electronic structure, and catalytic reactivity. J. Phys. Chem. A 2011, 115, 8255–8263. [Google Scholar] [CrossRef] [PubMed]
- Embs, J.P.; Burankova, T.; Reichert, E.; Hempelmann, R. Cation dynamics in the pyridinium based ionic liquid 1-N-butylpyridinium bis ((trifluoromethyl) sulfonyl) as seen by quasielastic neutron scattering. J. Phys. Chem. B 2012, 116, 13265–13271. [Google Scholar] [CrossRef] [PubMed]
- Zeinolabedin Hezave, A.; Dorostkar, S.; Ayatollahi, S.; Nabipour, S.; Hemmateenejad, B. Effect of different families (imidazolium and pyridinium) of ionic liquids-based surfactants on interfacial tension of water/crude oil system. Fluid Phase Equilib. 2013, 360, 139–145. [Google Scholar] [CrossRef]
- Sastry, N.V.; Vaghela, N.M.; Macwan, P.M.; Soni, S.S.; Aswal, V.K.; Gibaud, A. Aggregation behavior of pyridinium based ionic liquids in water—Surface tension, 1H NMR chemical shifts, SANS and SAXS measurements. J. Colloid Interface Sci. 2012, 371, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Harustiak, M.; Hronec, M.; Ilavsky, J.; Witek, S. Micellar catalysts in the CoBr2 catalyzed oxidation of p-xylene in water. Catal. Lett. 1998, 1, 391–393. [Google Scholar] [CrossRef]
- Sakaebe, H.; Matsumoto, H. N-Methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide (PP13–TFSI)–novel electrolyte base for Li battery. Electrochem. Commun. 2003, 5, 594–598. [Google Scholar] [CrossRef]
- Lethesh, K.C.; van Hecke, K.; van Meervelt, L.; Nockemann, P.; Kirchner, B.; Zahn, S.; Binnemans, K. Nitrile-functionalized pyridinium, pyrrolidinium, and piperidinium ionic liquids. J. Phys. Chem. B 2011, 115, 8424–8438. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hagiwara, R.; Ito, Y. Room-temperature ionic liquids with high conductivities and wide electrochemical windows N-Alkyl-N-methylpyrrolidinium and N-Alkyl-N-methylpiperidinium fluorohydrogenates. Electrochem. Solid-State Lett. 2004, 7, 41–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, X.; Wang, X.; Chen, X. Lyotropic liquid crystalline phases with a series of N-alkyl-N-methylpiperidinium bromides and water. J. Colloid Interface Sci. 2013, 389, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Milioto, S.; Causi, S.; de Lisi, R. Thermodynamic properties of some N-alkyl-N-methylpiperidinium chlorides and N-alkylpiperidine hydrochlorides in water. J. Solut. Chem. 1993, 22, 1–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, X.; Wang, X.; Huang, D.; Chen, X. Micelle formation by N-alkyl-N-methylpiperidinium bromide ionic liquids in aqueous solution. Colloids Surf. A 2012, 412, 90–95. [Google Scholar] [CrossRef]
- Walden, P. Molecular weight and electrical conductivity of several fused salts. Bull. Russ. Acad. Sci. 1914, 8, 405–422. [Google Scholar]
- Weingärtner, H.; Knocks, A.; Schrader, W.; Kaatze, U. Dielectric spectroscopy of the room temperature molten salt ethylammonium nitrate. J. Phys. Chem. A 2001, 105, 8646–8650. [Google Scholar] [CrossRef]
- Garlitz, J.A.; Summers, C.A.; Flowers, R.A.; Borgstahl, G.E. Ethylammonium nitrate: A protein crystallization reagent. Acta Crystallogr. Sect. D 1999, 55, 2037–2038. [Google Scholar] [CrossRef]
- Summers, C.A.; Flowers, R.A. Protein renaturation by the liquid organic salt ethylammonium nitrate. Protein Sci. 2000, 9, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Yamauchi, A.; Roman, R.; Casassa, E.Z. Micelle formation in ethylammonium nitrate, a low-melting fused salt. J. Colloid Interface Sci. 1982, 88, 89–96. [Google Scholar] [CrossRef]
- Evans, D.F.; Yamauchi, A.; Wei, G.J.; Bloomfield, V.A. Micelle size in ethylammonium nitrate as determined by classical and quasi-elastic light scattering. J. Phys. Chem. 1983, 87, 3537–3541. [Google Scholar] [CrossRef]
- Tamura-Lis, W.; Lis, L.J.; Quinn, P.J. Structures and mechanisms of lipid phase transitions in nonaqueous media: Dipalmitoylphosphatidylcholine in fused salt. J. Phys. Chem. 1987, 91, 4625–4627. [Google Scholar] [CrossRef]
- Zhao, M.W.; Gao, Y.A.; Zheng, L.Q. Liquid crystalline phases of the amphiphilic ionic liquid N-hexadecyl-N-methylpyrrolidinium bromide formed in the ionic liquid ethylammonium nitrate and in water. J. Phys. Chem. B 2010, 114, 11382–11389. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Kaler, E.W.; Benton, W.J. Liquid crystals in a fused salt: Beta, gamma distearoylphosphatidylcholine in N-ethylammonium nitrate. J. Phys. Chem. 1983, 87, 533–535. [Google Scholar] [CrossRef]
- Araos, M.U.; Warr, G.G. Self-assembly of nonionic surfactants into lyotropic liquid crystals in ethylammonium nitrate, a room-temperature ionic liquid. J. Phys. Chem. B 2005, 109, 14275–14277. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Chen, S.H.; Schriver, G.W.; Arnett, E.M. Thermodynamics of solution of nonpolar gases in a fused salt. Hydrophobic bonding behavior in a nonaqueous system. J. Am. Chem. Soc. 1981, 103, 481–482. [Google Scholar] [CrossRef]
- Kang, W.; Dong, B.; Gao, Y.; Zheng, L. Aggregation behavior of long-chain imidazolium ionic liquids in ethylammonium nitrate. Colloid Polym. Sci. 2010, 288, 1225–1232. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, M.; Zheng, L. Micelle formation by N-alkyl-N-methylpyrrolidinium bromide in ethylammonium nitrate. Colloids Surf. A 2011, 392, 305–312. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Drummond, C.J. Many protic ionic liquids mediate hydrocarbon-solvent interactions and promote amphiphile self-assembly. Langmuir 2007, 23, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Drummond, C.J. Formation of amphiphile self-assembly phases in protic ionic liquids. J. Phys. Chem. B 2007, 111, 4082–4088. [Google Scholar] [CrossRef] [PubMed]
- Thomaier, S.; Kunz, W. Aggregates in mixtures of ionic liquids. J. Mol. Liquids 2007, 130, 104–107. [Google Scholar] [CrossRef]
- Li, N.; Zhang, S.H.; Zheng, L.Q.; Dong, B.; Li, X.W.; Yu, L. Aggregation behavior of long-chain ionic liquids in an ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 4375–4377. [Google Scholar] [PubMed]
- Blesic, M.; Lopes, A.; Melo, E.; Petrovski, Z.; Plechkova, N.V.; Canongia Lopes, J.N.; Rebelo, L.P.N. On the self-aggregation and fluorescence quenching aptitude of surfactant ionic liquids. J. Phys. Chem. B 2008, 112, 8645–8650. [Google Scholar] [CrossRef] [PubMed]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Jaycock, M.J.; Parfitt, G.D. Chemistry of Interfaces; John Wiley: New York, NY, USA, 1981. [Google Scholar]
- Yang, C.; Chen, X.; Qiu, H.; Zhuang, W.; Chai, Y.; Hao, J. Dissipative particle dynamics simulation of phase behavior of aerosol OT/water system. J. Phys. Chem. B 2006, 110, 21735–21740. [Google Scholar] [CrossRef] [PubMed]
- Lava, K.; Binnemans, K.; Cardinaels, T. Piperidinium, piperazinium and morpholinium ionic liquid crystals. J. Phys. Chem. B 2009, 113, 9506–9511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Du, M.; Liu, Y.; Wang, S.; Zhao, J.; Chen, A.; Peng, D.; Zhao, M. Aggregation Behavior of Long-Chain Piperidinium Ionic Liquids in Ethylammonium Nitrate. Molecules 2014, 19, 20157-20169. https://doi.org/10.3390/molecules191220157
Dai C, Du M, Liu Y, Wang S, Zhao J, Chen A, Peng D, Zhao M. Aggregation Behavior of Long-Chain Piperidinium Ionic Liquids in Ethylammonium Nitrate. Molecules. 2014; 19(12):20157-20169. https://doi.org/10.3390/molecules191220157
Chicago/Turabian StyleDai, Caili, Mingyong Du, Yifei Liu, Shilu Wang, Jianhui Zhao, Ang Chen, Dongxu Peng, and Mingwei Zhao. 2014. "Aggregation Behavior of Long-Chain Piperidinium Ionic Liquids in Ethylammonium Nitrate" Molecules 19, no. 12: 20157-20169. https://doi.org/10.3390/molecules191220157