Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
| Compounds | Essential Oils (% ± SD) | Identification Methods | ||||||
|---|---|---|---|---|---|---|---|---|
| LRIHP5 | Garlic | Onion | Leek | Chinese Chive | Shallot | Chive | ||
| Isoamyl alcohol | 762 | - | 0.13 ± 0.03 | - | - | - | - | SM, LRI, Std |
| Methyl 1-propenyl sulfide a | 764 | - | - | - | - | tr | - | SM, LRI |
| Dimethyl disulfide | 767 | 1.12 ± 0.18 | tr | 0.28 ± 0.02 | 19.58 ± 2.62 | tr | tr | SM, LRI, Std |
| (Z)-3-hexenal | 769 | 0.15 ± 0.01 | - | tr | tr | 0.16 ± 0.03 | tr | SM, LRI, Std |
| 2-methylpentenal a | 776 | 0.15 ± 0.02 | - | - | - | 0.51 ± 0.07 | - | SM, LRI |
| Hexanal | 802 | - | - | tr | tr | 0.17 ± 0.02 | - | SM, LRI, Std |
| Propanal diethyl acetal t | 813 | - | - | tr | - | - | 0.16 ± 0.03 | SM |
| 2-Ethylpyridine | 836 | 0.10 ± 0.01 | - | - | - | - | - | SM, LRI |
| (E)-hexenol | 848 | - | - | tr | - | - | - | SM, LRI, Std |
| 1,3-Propanedithiol t | 851 | - | - | tr | - | - | 0.17 ± 0.01 | SM |
| Diallyl sulfide | 854 | 6.59 ± 0.55 | tr | tr | 1.47 ± 0.10 | tr | tr | SM, LRI, Std |
| n-Hexanol | 866 | - | - | 0.25 ± 0.02 | - | - | - | SM, LRI, Std |
| Allyl propyl sulfide | 867 | 0.09 ± 0.01 | - | 0.15 ± 0.01 | - | 0.16 ± 0.03 | - | SM, LRI |
| Bis-(1-propenyl)-sulfide a | 884 | 0.08 ± 0.01 | - | - | 0.21 ± 0.01 | - | - | SM, LRI |
| Dimethyl thiophene a | 871 | - | - | - | - | - | 0.15 ± 0.01 | SM, LRI |
| Nonane | 900 | - | - | - | - | - | tr | SM, LRI, Std |
| Dimethyl thiophene a | 902 | 0.08 ± 0.01 | 0.18 ± 0.01 | 0.34 ± 0.03 | - | 0.21 ± 0.01 | 0.26 ± 0.01 | SM, LRI |
| Allyl methyl disulfide | 915 | 3.69 ± 0.02 | - | - | 14.37 ± 0.36 | - | - | SM, LRI, Std |
| Methyl propyl disulfide | 926 | 0.25 ± 0.01 | 2.11 ± 0.16 | 4.48 ± 0.33 | 1.21 | 3.26 ± 0.27 | 2.55 ± 0.13 | SM, LRI |
| Methyl 1-propenyl disulfide a | 934 | 0.46 ± 0.02 | 0.51 ± 0.04 | 0.27 ± 0.03 | 6.07 ± 0.13 | 1.33 ± 0.13 | 0.62 ± 0.03 | SM, LRI |
| Dimethyl trisulfide | 962 | 0.33 ± 0.01 | 0.30 ± 0.01 | 0.12 ± 0.01 | 14.34 ± 0.05 | 1.22 ± 0.06 | 0.65 ± 0.02 | SM, LRI, Std |
| 2-Pentylfuran | 990 | - | - | - | - | - | 0.14 ± 0.01 | SM, LRI |
| Diallyl disulfide | 1084 | 37.90 ± 0.07 | - | - | 5.14 ± 0.21 | 0.13 | - | SM, LRI, Std |
| Allyl propyl disulfide | 1088 | - | 0.42 ± 0.08 | 0.73 ± 0.04 | - | 0.55 ± 0.06 | 0.44 ± 0.02 | SM, LRI |
| Linalool | 1101 | - | - | - | 1.75 ± 0.14 | - | - | SM, LRI, Std |
| Dipropyl disulfide | 1105 | 0.25 ± 0.06 | 30.92 ± 0.03 | 47.70 ± 0.03 | 1.24 ± 0.05 | 15.17 ± 0.18 | 19.49 ± 0.08 | SM, LRI |
| Ethyl-3-(methylthio)propionate | 1113 | 0.09 ± 0.01 | - | - | - | - | - | SM, LRI |
| 1-Propenyl propyl disulfide a | 1116 | - | 7.26 ± 0.06 | 3.75 ± 0.02 | - | 4.57 ± 0.05 | 5.84 ± 0.05 | SM, LRI |
| 2,4,5-tTrithiahexane t | 1118 | - | - | - | 0.15 ± 0.01 | - | - | SM |
| 3,5-Dimethyl-1,2,4-trithiolane | 1126 | - | 0.12 ± 0.01 | 0.21 ± 0.01 | - | - | - | SM, LRI |
| Allyl methyl trisulfide | 1131 | 7.26 ± 0.05 | - | - | 7.24 ± 0.38 | - | 0.2 | SM, LRI |
| Menthone | 1145 | - | - | - | 1.91 ± 0.12 | - | - | SM, LRI, Std |
| Methyl propyl trisulfide | 1148 | - | 5.20 ± 0.02 | 3.19 ± 0.02 | - | 9.20 ± 0.10 | 8.47 ± 0.10 | SM, LRI |
| Methyl 1-propenyl trisulfide | 1153 | - | 0.34 ± 0.01 | 0.13 ± 0.01 | - | 0.50 ± 0.08 | 0.36 ± 0.01 | SM, LRI |
| Methyl-1-(methylthio)ethyl-disulfide t | 1159 | 0.47 ± 0.05 | 0.15 ± 0.01 | - | 0.68 ± 0.05 | 0.51 ± 0.02 | SM | |
| n-Nonanol | 1172 | - | 0.47 ± 0.01 | - | - | 0.15 ± 0.01 | SM, LRI, Std | |
| 3,4-Dihydro-3-vinyl-1,2-dithiin t | 1177 | 0.13 ± 0.02 | - | - | - | - | - | SM |
| Borneol | 1183 | - | - | - | - | 0.33 ± 0.01 | 0.85 ± 0.01 | SM, LRI, Std |
| α-Terpineol | 1187 | - | - | - | 0.32 ± 0.01 | - | - | SM, LRI, Std |
| Methyl salicylate | 1189 | - | - | - | 0.46 ± 0.01 | - | - | SM, LRI, Std |
| Methyl chavicol | 1196 | - | - | - | - | 0.11 ± 0.01 | - | SM, LRI, Std |
| Dimethyl tetrasulfide | 1206 | 0.56 ± 0.01 | 0.15 ± 0.01 | - | 2.82 ± 0.19 | 0.46 ± 0.01 | 0.39 ± 0.01 | SM, LRI, Std |
| 3-Ethyl-5-methyl-1,2,4-trithiolane a, t | 1216 | - | 0.13 ± 0.01 | - | - | 0.11 ± 0.01 | - | SM |
| 3-Ethyl-5-methyl-1,2,4-trithiolane a, t | 1219 | - | 0.16 ± 0.01 | 0.54 ± 0.01 | - | 0.17 ± 0.01 | - | SM |
| Butyl thiocyante | 1239 | - | - | - | - | 0.21 ± 0.01 | - | SM, LRI |
| Methyl 1-(methylthiopropyl) disulfide | 1249 | - | 0.36 ± 0.04 | 0.12 ± 0.01 | 0.59 ± 0.04 | 0.52 ± 0.06 | 0.20 ± 0.01 | SM, LRI |
| 2-Undecanone | 1292 | - | 0.53 ± 0.01 | - | 0.62 ± 0.04 | 0.82 ± 0.01 | 0.10 ± 0.01 | SM, LRI |
| Tridecane | 1301 | - | 0.49 ± 0.05 | - | - | - | - | SM, LRI, Std |
| Diallyl trisulfide | 1305 | 28.06 ± 0.63 | - | - | - | - | - | SM, LRI |
| 3-Methoxyoctane t | 1311 | 1.10 ± 0.03 | - | - | - | 0.88 ± 0.01 | 1.24 ± 0.02 | SM |
| Dipropyl trisulfide | 1328 | tr | 17.10 ± 0.28 | 15.01 ± 0.27 | tr | 11.14 ± 0.14 | 15.21 ± 0.18 | SM, LRI |
| 1-Propenyl propyl trisulfide a | 1332 | - | - | 0.43 ± 0.02 | - | 1.36 ± 0.02 | - | SM, LRI |
| Allyl propyl trisulfide | 1334 | - | 1.84 ± 0.01 | 1.58 ± 0.03 | - | 1.97 ± 0.01 | 1.92 ± 0.02 | SM, LRI |
| Di-1-propenyl trisulfide | 1347 | 0.23 ± 0.05 | 3.07 ± 0.01 | - | - | 0.26 ± 0.02 | 0.14 ± 0.01 | SM, LRI |
| Eugenol | 1356 | 0.21 ± 0.01 | - | - | 0.26 0.01 | - | - | SM, LRI |
| Benzyl thiocyanate | 1359 | - | - | 0.20 ± 0.02 | 0.27 ± 0.02 | - | - | SM, LRI, Std |
| α-Copaene | 1370 | - | - | - | - | - | 0.14 ± 0.01 | SM, LRI, Std |
| Allyl methyl tetrasulfide | 1371 | 1.07 ± 0.03 | - | - | 1.21 ± 0.09 | - | - | SM, LRI |
| Benzyl methyl disulfide t | 1373 | - | - | - | 0.37 ± 0.04 | - | - | SM |
| Geranyl acetate | 1386 | - | - | 0.83 ± 0.01 | - | - | - | SM, LRI |
| Methyl eugenol | 1405 | - | - | - | - | 0.14 ± 0.03 | - | SM, LRI, Std |
| 6,10-Dimethyl 2-undecanone | 1406 | - | - | - | - | - | 0.32 ± 0.01 | SM, LRI |
| β-Caryophyllene | 1410 | - | - | - | - | - | 0.17 ± 0.01 | SM, LRI, Std |
| 3,6-Dimethyl-2,4,5,7 tetrathioctane t | 1423 | - | - | 0.39 ± 0.04 | - | - | 0.11 ± 0.01 | SM |
| 2-Hexyl-5-methyl 3(2H)-furanone | 1440 | - | 1.26 ± 0.01 | 0.13 ± 0.01 | - | 5.40 ± 0.15 | 0.15 ± 0.01 | SM, LRI |
| β-Selinene | 1445 | - | - | - | - | - | 0.25 ± 0.03 | SM, LRI |
| (E)-β-Farnesene | 1457 | - | - | - | - | - | 1.50 ± 0.06 | SM, LRI |
| β-Ionone | 1481 | - | - | - | 0.13 ± 0.01 | - | 0.30 ± 0.04 | SM, LRI, Std |
| Methyl-1-propylthioethyl tetrasulfide | 1487 | - | - | 0.43 ± 0.01 | - | - | - | SM, LRI |
| 2-Tridecanone | 1496 | - | 0.32 ± 0.03 | - | - | 0.63 ± 0.01 | - | SM, LRI |
| γ-Cadinene | 1506 | 0.10 ± 0.01 | - | - | - | - | - | SM, LRI |
| α-Farnesene | 1509 | - | - | - | - | - | 2.56 ± 0.08 | SM, LRI |
| β-Sesquiphellandrene | 1521 | - | - | - | - | - | 0.15 ± 0.01 | SM, LRI |
| Diallyl tetrasulfide | 1538 | 4.14 ± 0.11 | - | - | 0.22 ± 0.02 | - | - | SM, LRI, Std |
| 2-Methyl-3,4-dithiaheptane | 1558 | - | 6.48 ± 0.08 | 2.03 ± 0.04 | - | 4.42 ± 0.05 | 3.70 ± 0.05 | SM, LRI |
| Dipropyl tetrasulfide | 1573 | - | 0.55 ± 0.04 | - | - | - | 0.35 ± 0.01 | SM, LRI |
| Tetradecanal | 1607 | - | - | 0.15 ± 0.02 | - | - | - | SM, LRI |
| Dimethyl pentasulfide | 1686 | - | - | - | 0.23 ± 0.02 | - | - | SM, LRI |
| Propyl-1-(propylthio)ethyl trisulfide t | 1700 | - | - | 0.28 ± 0.01 | - | 0.44 ± 0.03 | 1.12 ±0.02 | SM |
| 6,10,14-Trimethyl-2-pentadecanone | 1845 | - | - | 0.52 ± 0.01 | 0.16 ± 0.01 | 0.23 ± 0.01 | 1.35 ± 0.01 | SM, LRI |
| Benzyl salicylate | 1857 | - | - | - | - | 0.27 ± 0.01 | - | SM, LRI |
| 2,4-Diméthyl-5,6-dithia-2,7-nonadienal | 1885 | 0.44 ± 0.02 | - | - | - | - | - | SM, LRI |
| Methyl palmitate | 1928 | - | 0.81 ± 0.04 | 0.17 ± 0.01 | 0.15 ± 0.01 | 1.39 ± 0.02 | 0.30 ± 0.01 | SM, LRI, Std |
| Palmitic acid | 1970 | - | - | 0.76 ± 0.01 | 1.58 ± 0.10 | - | 2,17 ± 0.06 | SM, LRI, Std |
| Ethyl palmitate | 1996 | - | 0.42 ± 0.02 | 0.48 ± 0.01 | 0.41 ± 0.03 | 0.43 ± 0.01 | 0.56 ± 0.01 | SM, LRI, Std |
| Methyl linoleate | 2093 | - | 0.55 ± 0.01 | - | 0.14 ± 0.01 | - | 0.25 ± 0.01 | SM, LRI, Std |
| Methyl linolenate | 2099 | - | - | - | 0.16 ± 0.01 | - | - | SM, LRI, Std |
| Phytol | 2113 | - | - | 0.16 ± 0.01 | 0.61 ± 0.03 | - | - | SM, LRI |
| Ethyl linoleate | 2161 | - | - | 0.26 ± 0.01 | 0.21 ± 0.01 | 0.42 ± 0.04 | 0.35 ± 0.01 | SM, LRI, Std |
| Ethyl oleate | 2167 | - | 0.18 ± 0.01 | - | - | 0.36 ± 0.01 | - | SM, LRI, Std |
| Ethyl linolenate | 2168 | - | - | 0.21 ± 0.01 | - | - | 0.35 ± 0.02 | SM, LRI |
| Ethyl α-linolénate | 2244 | - | - | - | 0.19 ± 0.02 | - | - | SM, LRI |
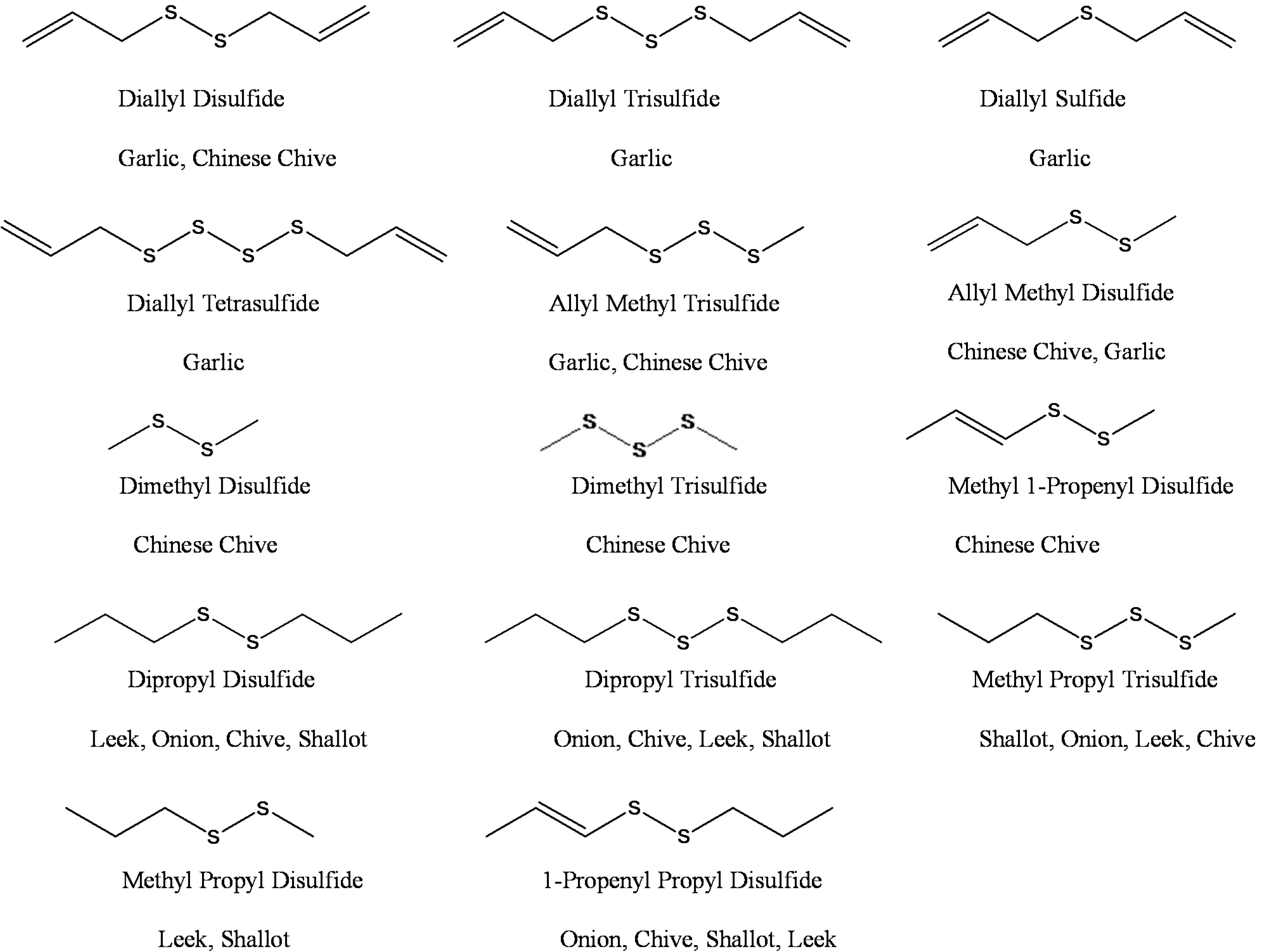
2.2. In Vitro Antimicrobial Activity
| Pathogens | Inhibition Diameter (mm) 2 Including Disk Diameter of 6.0 mm | |||
|---|---|---|---|---|
| Garlic | Onion | Leek | ||
| Staphylococus aureus | 20.0 ± 0.0 a | 15.5 ± 2.1 a | 10.0 ± 0.0 a | |
| Salmonella Typhimurium | 9.0 ± 1.0 c | 12.0 ± 1.8 a b | 6 mm | |
| Listeria monocytogenes | 23.0 ± 1.4 a | 15.0 ± 1.4 a | 6 mm | |
| Escherichia coli | 9.3 ± 0.9 c | 6 mm | 6 mm | |
| Campylobacter jejuni | 12.6 ± 2.1 b | 9.0 ± 1.2 b | 9.3 ± 1.9 a | |
| Pathogens | Inhibition Diameter (mm) 2 Including Disk Diameter of 6.0 mm | |||
| Chinese Chive | Shallot | Chive | Positive Control | |
| Staphylococus aureus | 18.5 ± 0.7 a | 20.0 ± 0.1 a | 11.5 ± 0.7 a | 30.6 ± 0.6 a |
| Salmonella Typhimurium | 9.3 ± 1.2 b | 11.3 ± 2.3 b | 6 mm | 25.6 ± 1.3 b |
| Listeria monocytogenes | 6 mm | 6 mm | 6 mm | 28.0 ± 0.5 a b |
| Escherichia coli | 9.0 ± 1.4 b | 6 mm | 6 mm | 25.5 ± 1.1 b |
| Campylobacter jejuni | 21.0 ± 1.7 a | 11.6 ±1.5 b | ±2.1 a | 25.3 ± 1.2 b |
2.3. Total Phenolic Content
| Essential Oils | Total Phenol Contents GAE * (mg/g) |
|---|---|
| Garlic | 5.61 ± 0.69 c |
| Onion | 3.29 ± 0.12 d |
| Leek | 10.79 ± 0.53 b |
| Chinese chive | 4.24 ± 0.11 d |
| Shallot | 11.14 ± 0.43 b |
| Chive | 6.76 ± 0.37 c |
| BHT | 46.77 ± 0.81 a |
2.4. DPPH Radical Scavenging Activity
| Essential Oils | DPPH Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|
| 2 mg/mL | 4 mg/mL | 8 mg/mL | 12 mg/mL | 16 mg/mL | 20 mg/mL | IC50 * | |
| Garlic | 31.35 ± 1.91 | 37.93 ± 1.59 | 51.07 ± 0.67 | 64.22 ± 0.78 | 77.37 ± 0.79 | 90.52 ± 0.59 | 7.67 |
| Onion | 22.30 ± 0.97 | 25.34 ± 0.19 | 31.43 ± 1.60 | 37.52 ± 0.32 | 43.61 ± 0.42 | 49.70 ± 1.49 | 20.19 |
| Chinese chive | 17.93 ± 0.89 | 24.24 ± 1.39 | 36.85 ± 1.38 | 49.46 ± 0.30 | 62.08 ± 0.68 | 74.69 ± 0.31 | 12.16 |
| Chive | 39.05 ± 0.33 | 45.15 ± 0.11 | 57.34 ± 1.14 | 69.54 ± 1.45 | 81.73 ± 0.11 | 93.93 ± 1.70 | 5.59 |
| 1 mg/mL | 2 mg/mL | 3 mg/mL | 4 mg/mL | 5 mg/mL | |||
| Leek | 9.90 ± 1.32 | 21.37 ± 1.70 | 32.84 ± 1.46 | 44.31 ± 0.64 | 55.78 ± 1.49 | 4.49 | |
| Shallot | 29.95 ± 1.18 | 42.59 ± 1.11 | 51.99 ± 1.44 | 61.38 ± 1.58 | 70.77 ± 1.64 | 2.70 | |
| 0.01 mg/mL | 0.02 mg/mL | 0.04 mg/mL | 0.06 mg/mL | 0.08 mg/mL | |||
| BHT | 16.01 ± 1.71 | 35.3 ± 1.27 | 68.93 ± 2.01 | 85.50 ± 0.13 | 91.21 ± 0.41 | 0.03 | |
2.5. Heating Test
3. Experimental Section
3.1. Plant Materials, Chemicals and Standards
3.2. Essential Oils: Extraction and Yield

3.3. GC-FID and GC-MS Identification
3.4. Bacterial Strains
3.5. Screening for Antibacterial Activity
3.6. Folin-Ciocalteu Assay
3.7. DPPH Free-Radical-Scavenging Assay
3.8. Frying Oil Test
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tepe, B.; Sokmen, M.; Akpulat, H.; Sokmen, A. In vitro antioxidant activities of the methanol extracts of five species from Turkey. Food Chem. 2005, 92, 89–92. [Google Scholar] [CrossRef]
- Benkeblia, N.; Lanzotti, V. Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Food 2007, 1, 193–201. [Google Scholar]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corzomartinez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Ielsch, V. Antimicrobial effects of garlic, clove and red hot chilli on Listeria monocytogenes in broth model systems and soft cheese. Int. J. Food Sci. Nutr. 2003, 54, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Mima, T.; Ohnishi, S.T.; Mori, K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000, 66, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Cheng, W. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003, 63, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Cheng, W. Antioxidant activity of several Allium members. J. Agric. Food Chem. 1998, 46, 4097–4101. [Google Scholar] [CrossRef]
- Iranshahi, M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Kim, J.W.; Huh, J.E.; Kyung, S.H.; Kyung, K.H. Antimicrobial activity of alk(en)yl sulfides found in essential oils of garlic and onion. Food Sci. Biotechnol. 2004, 13, 235–239. [Google Scholar]
- Tsao, S.; Yin, M. In vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 2001, 50, 646–649. [Google Scholar] [PubMed]
- Amagase, H.; Petesch, B.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [PubMed]
- Ye, C.L.; Dai, D.H.; Hu, W.L. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenis bacteria of chives (Allium schoenoprasum). Bionsci. Biotechnol. Biochem. 2008, 72, 2987–2991. [Google Scholar] [CrossRef]
- Amin, M.; Kapadnis, B.P. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Ind. J. Exp. Biol. 2005, 43, 751–754. [Google Scholar]
- Bernaert, N.; de Paepe, D.; Bouten, C.; de Clercq, H.; Stewart, D.; van Bockstaele, E.; de Loose, M.; van Droogenbroeck, B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fista, G.; Bloukas, J.; Siomos, A. Effect of leek and onion on processing and quality characteristics of Greek traditional sausages. Meat Sci. 2004, 68, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 2006, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Stajner, D.; Milic-DeMarino, M.; Canadanovic-Brunet, J. Screening for antioxidant properties of leeks, Allium sphaerocephalon L. J. Herbs Spices Med. Plants 2003, 10, 75–82. [Google Scholar] [CrossRef]
- Yabuki, Y.; Mukaida, Y.; Saito, Y.; Oshima, K.; Takahashi, T.; Muroi, E.; Hashimoto, K.; Uda, Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem. 2010, 120, 343–348. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Shallot (Allium ascalonicum L.) oil: Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria. Afr. J. Microbiol. Res. 2009, 3, 747–750. [Google Scholar]
- Banerjee, S.; Mukherjee, K.; Maulik, S. Garlic as an Antioxidant: The Good, The Bad and The Ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The Role of Diallyl Sulfides and Dipropyl Sulfides in the In Vitro Antimicrobial Activity of the Essential Oil of Garlic, Allium sativum L., and Leek, Allium porrum L. Phytother. Res. 2012, 27, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.G.; Fritz, R.; del Valle, C.E.; Roura, S.I. Antimicrobial activity of essential oils on native microbial population of organic Swiss chard. Lebensm. Wiss. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Babu, A.J.; Sundari, A.R.; Indumathi, J.; Srujan, R.V.N.; Sravanthi, M. Study on the Antimicrobial activity and Minimum Inhibitory Concentration of Essential Oils of Spices. Vet. World 2011, 4, 311–316. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT—Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Razavi Rohani, S.M.; Moradi, M.; Mehdizadeh, T.; Saei-Dehkordi, S.S.; Griffiths, M.W. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. LWT—Food Sci. Technol. 2011, 44, 2260–2265. [Google Scholar] [CrossRef]
- Zohri, A.N.; Abdel-Gawad, K.; Saber, S. Antibacterial, antidermatophytic and antitoxigenic activities of onion (Allium cepa L.) oil. Microbiol. Res. 1995, 150, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Kyung, K. Inhibitory activity of essential oils of garlic and onion against bacteria and yeasts. J. Food Prot. 2004, 67, 499–504. [Google Scholar] [PubMed]
- O’Gara, E.; Hill, D.; Maslin, D. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.; Skandamis, P.; Coote, P.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oils, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Wilkinson, S. An overview of microbial lipids. In Microbial Lipids; Ratledge, C., Wilkinson, S.G., Eds.; Academic Press: London, UK, 1988; Volume 1, p. 3. [Google Scholar]
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kyung, K.H. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; Abd ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.; Deans, S. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Holley, R.; Patel, D. Improvement of shelflife and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Lampe, J. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999, 70, 475S–490S. [Google Scholar] [PubMed]
- Wangcharoen, W.; Morasuk, W. Effect of heat treatment on the antioxidant capacity of Garlic. Maejo Int. J. Sci. Technol. 2009, 3, 60–70. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Isitec Lab-Seppal, Indice de Folin/Polyphenols totaux; ISITEC: Montauban, France, 2013.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family. Molecules 2014, 19, 20034-20053. https://doi.org/10.3390/molecules191220034
Mnayer D, Fabiano-Tixier A-S, Petitcolas E, Hamieh T, Nehme N, Ferrant C, Fernandez X, Chemat F. Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family. Molecules. 2014; 19(12):20034-20053. https://doi.org/10.3390/molecules191220034
Chicago/Turabian StyleMnayer, Dima, Anne-Sylvie Fabiano-Tixier, Emmanuel Petitcolas, Tayssir Hamieh, Nancy Nehme, Christine Ferrant, Xavier Fernandez, and Farid Chemat. 2014. "Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family" Molecules 19, no. 12: 20034-20053. https://doi.org/10.3390/molecules191220034









