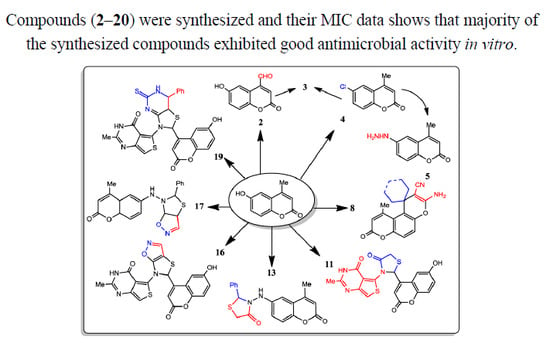
Microwave-Assisted Synthesis of Novel 2H-Chromene Derivatives Bearing Phenylthiazolidinones and Their Biological Activity Assessment
Abstract
:1. Introduction
2. Results and Discussion
Chemistry
| Compounds | Mol. Formula | Mol. Wt. | Time (min/h) | Yield (%) | Melting Point (°C) | ||
|---|---|---|---|---|---|---|---|
| Microwave(min) | Conventional(h) | Microwave | Conventional | ||||
| 2 | C10H6O4 | 190.15 | 8 | 5 | 97 | 85 | 217–219 |
| 3 | C10H5ClO3 | 208.60 | - | 1 | 96 | 75 | 145–147 |
| 4 | C10H7ClO2 | 194.61 | - | 1 | - | 80 | 236–238 |
| 5 | C10H10N2O2 | 190.20 | 9 | 4 | 97 | 85 | 126–128 |
| 8a | C18H16N2O3 | 308.12 | 9 | 4 | 98 | 78 | 167–169 |
| 8b | C19H18N2O3 | 322.36 | 10 | 5 | 95 | 82 | 151–153 |
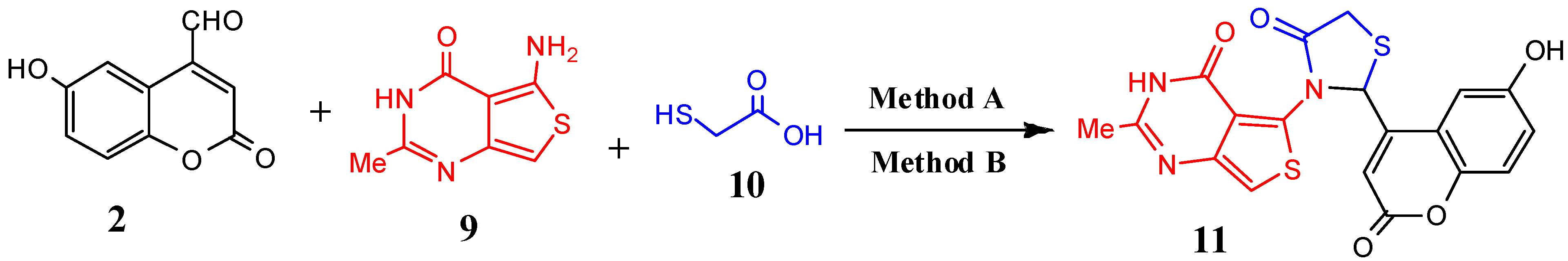
| 11 | C19H13N3O5S2 | 427.45 | 9 | 7 | 96 | 89 | 251–253 |
| 13 | C19H16N2O3S | 352.41 | 8 | 4 | 98 | 80 | 278–280 |
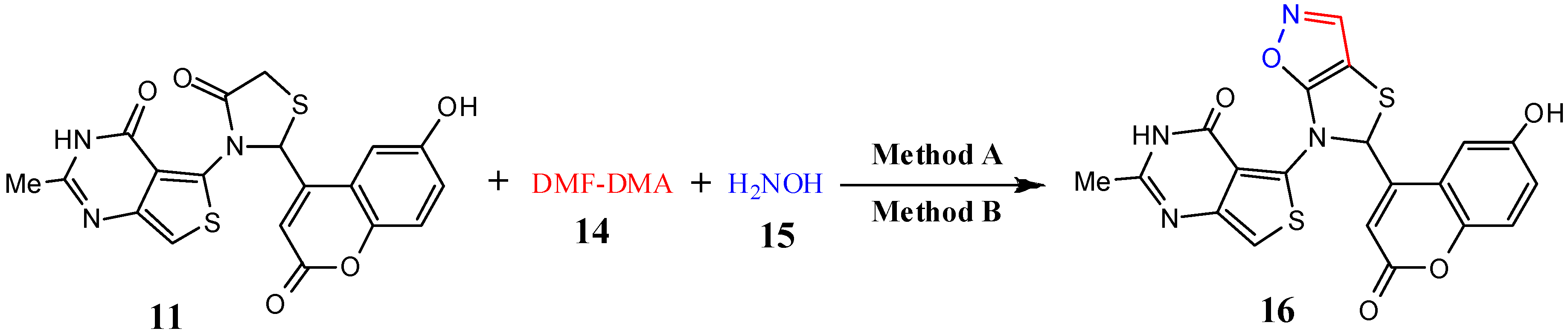
| 16 | C20H12N2O5S2 | 452.46 | 10 | 5 | 97 | 70 | 211–213 |
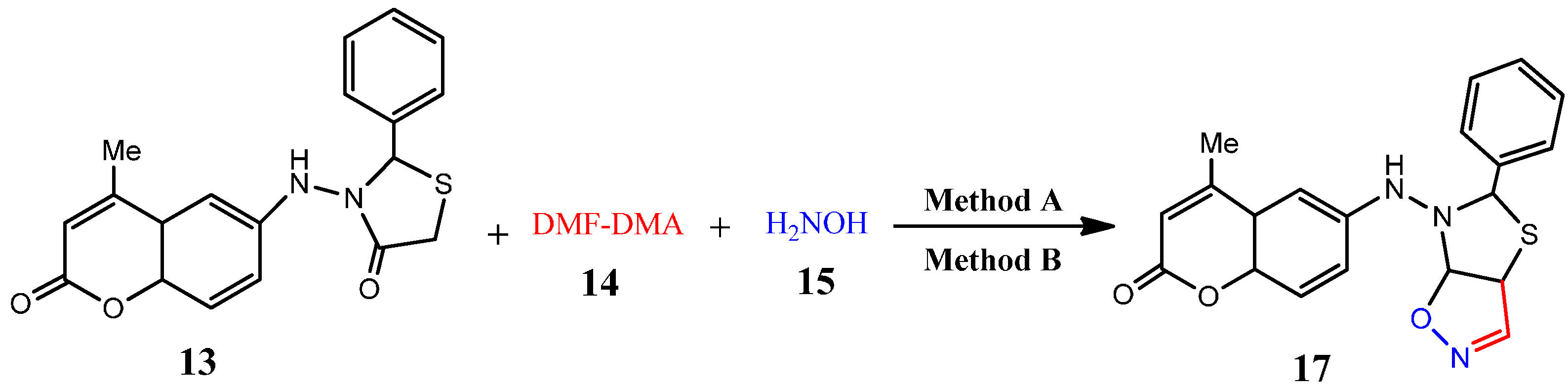
| 17 | C20H15N3O3S | 377.42 | 8 | 6 | 96 | 80 | 182–184 |
| 19 | C27H19N5O4S3 | 573.67 | 10 | 6 | 96 | 73 | 198–200 |
| 20 | C27H22N4O2S2 | 498.62 | 10 | 7 | 95 | 85 | 217–219 |



3. Experimental
3.1. General Information
3.2. Synthesis
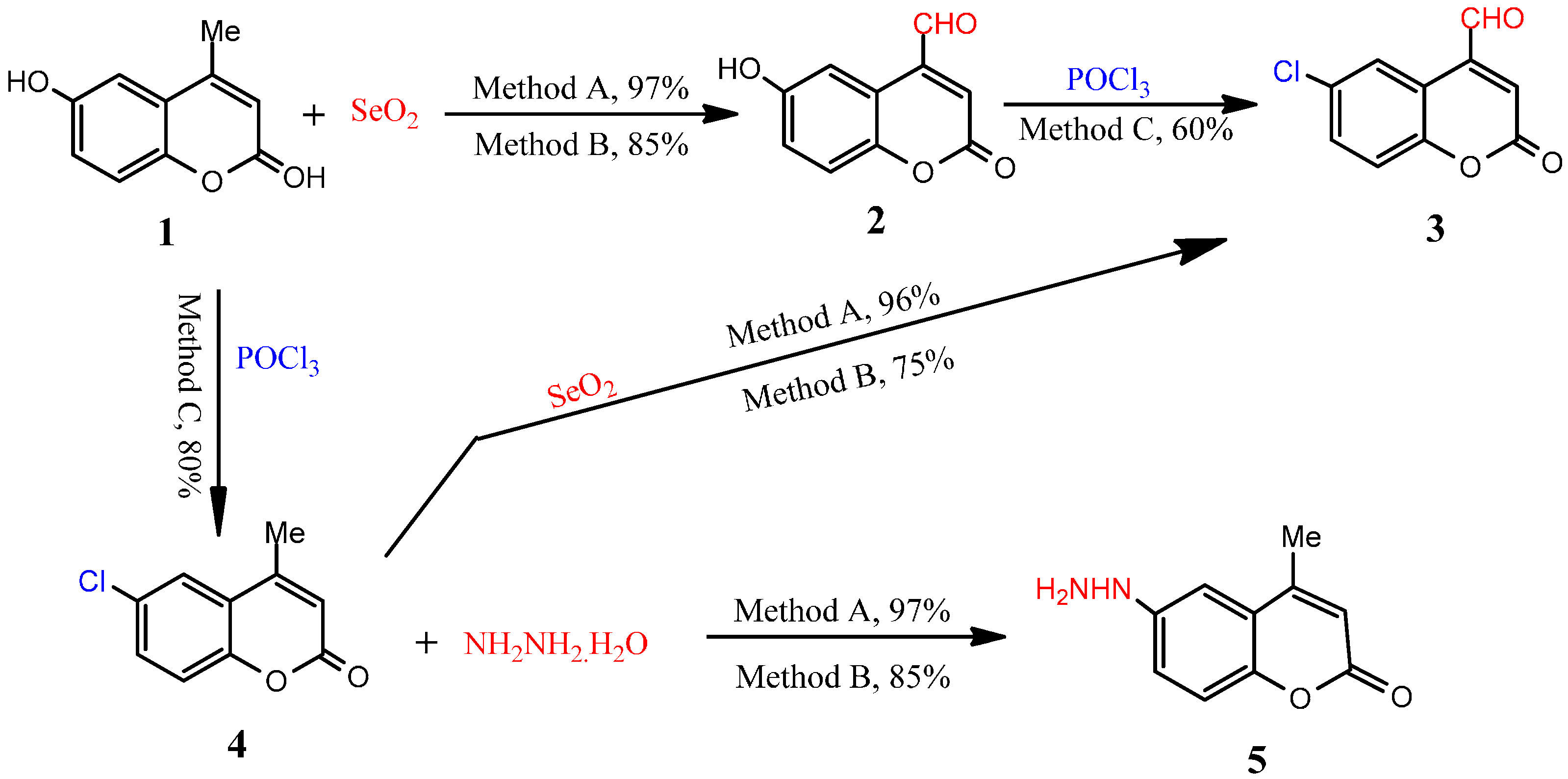
3.2.1. General Procedure for Microwave-Assisted Synthesis of 6-Hydroxy-2-oxo-2H-chromene-4-carbaldehyde (2), Method A
3.2.2. General Procedure for Conventional Synthesis of 6-Hydroxy-2-oxo-2H-chromene-4-carbaldehyde (2), Method B
3.2.3. Synthesis of 6-Chloro-2-oxo-2H-chromene-4-carbaldehyde (3)
Microwave-Assisted Synthesis, Method A
Conventional Synthesis, Method B
Conventional Synthesis, Method C
3.2.4. Synthesis of 6-Chloro-4-methyl-2H-chromen-2-one (4), Method C
3.2.5. Synthesis of 6-Hydrazinyl-4-methyl-2H-chromen-2-one (5)
Microwave-Assisted Synthesis, Method A
Conventional Synthesis of 6-Hydrazinyl-4-methyl-2H-chromen-2-one (5), Method B
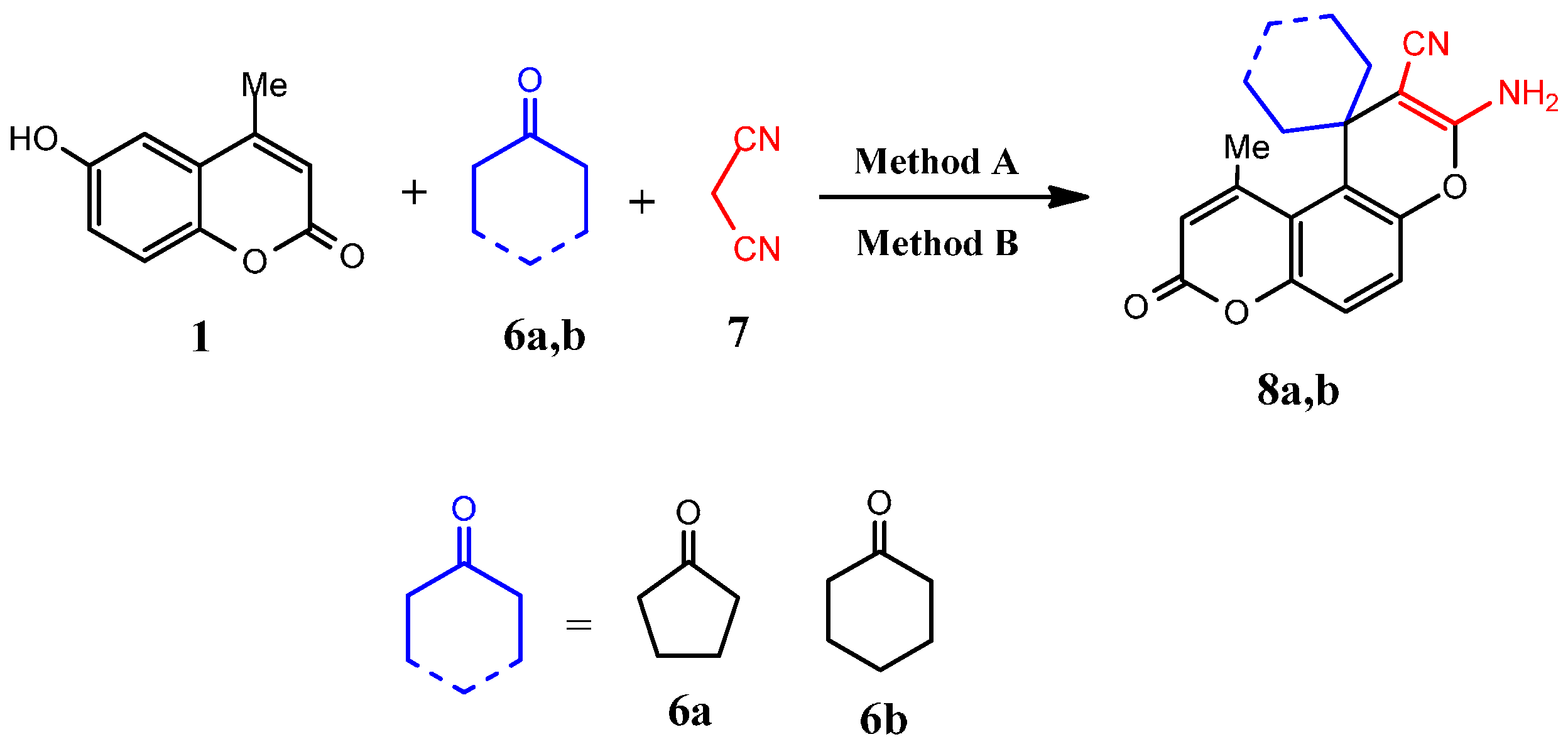
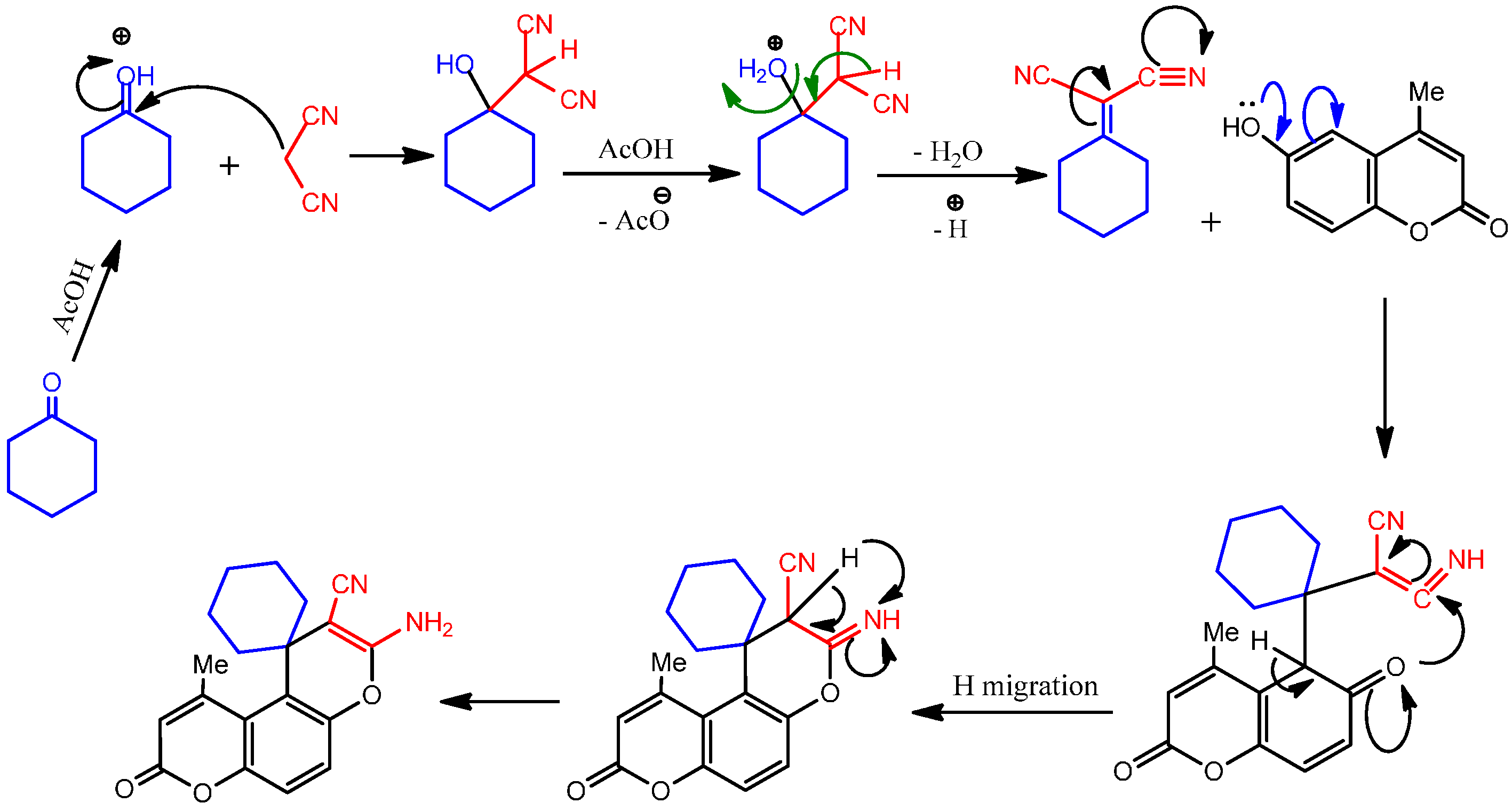
3.2.6. General Procedure for Microwave-Assisted Synthesis of Spiro Compounds 8a and 8b, Method A
3.2.7. General Procedure for Conventional Synthesis of Spiro Compounds 8a and 8b, Method B
3.2.8. Microwave-Assisted Synthesis of 5-(2-(6-Hydroxy-2-oxo-2H-chromen-4-yl)-4-oxothiazolidin-3-yl)-2-methylthieno[3,4-d]pyrimidin-4(3H)-one (11)
3.2.9. Conventional Synthesis of Compound 11
3.2.10. Microwave-Assisted Synthesis of 3-((4-Methyl-2-oxo-2H-chromen-6-yl)amino)-2-phenyl-thiazolidin-4-one (13)
3.2.11. Conventional Synthesis of Compound 13
3.2.12. General Procedure for Microwave-Assisted Synthesis of Thiazolo[5,4-d]isoxazole Derivatives 16 and 17, Method A
3.2.13. General Procedure for Conventional Synthesis of Synthesis of Thiazolo[5,4-d]isoxazole Derivatives 16 and 17, Method B
3.2.14. General Procedure for Microwave-Assisted Synthesis of Thiazolo[4,5-d]pyrimidine Derivatives 19 and 20, Method A
3.2.15. General Procedure for Conventional Synthesis of Thiazolo[5,4-d]isoxazole Derivatives 19 and 20, Method B
3.3. Antimicrobial Evaluation
| Compound | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungal Species | |||||
|---|---|---|---|---|---|---|---|---|
| (BS) | (CT) | (SP) | (EC) | (ST) | (VC) | (AF) | (CA) | |
| 1 | 500 | 500 | 500 | 250 | 500 | 500 | 1000 | >1000 |
| 2 | 250 | 500 | 250 | 500 | 500 | 100 | 500 | 100 |
| 3 | 1000 | 100 | 500 | 250 | 500 | 200 | 250 | 100 |
| 4 | 250 | 200 | 250 | 500 | 250 | 200 | 500 | 250 |
| 5 | 500 | 200 | 500 | 250 | 250 | 200 | 500 | 500 |
| 8a | 500 | 200 | 500 | 100 | 500 | 250 | 250 | 250 |
| 8b | 250 | 500 | 250 | 100 | 100 | 250 | 1000 | 500 |
| 11 | 500 | 100 | 500 | 250 | 65.5 | 250 | 1000 | 1000 |
| 13 | 250 | 200 | 250 | 250 | 250 | 200 | 500 | 250 |
| 16 | 500 | 500 | 50 | 250 | 500 | 500 | 1000 | 500 |
| 17 | 65.5 | 100 | 250 | 100 | 65.5 | 200 | 1000 | 1000 |
| 19 | 500 | 100 | 500 | 200 | 500 | 200 | 500 | 500 |
| 20 | 250 | 250 | 500 | 100 | 65.5 | 250 | 250 | 250 |
| A | 250 | 250 | 100 | 100 | 100 | 100 | 0 | 0 |
| B | 50 | 100 | 50 | 25 | 25 | 25 | 0 | 0 |
| C | 100 | 50 | 10 | 10 | 10 | 10 | 0 | 0 |
| D | 50 | 50 | 50 | 50 | 50 | 50 | 0 | 0 |
| E | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 500 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Domling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, D.; Lamour, V.; Tsvetkov, P.; Markov, A.A.; Deprez, M.; Klich, P.; Moras, D.; Briand, C.; Gilli, R. DNA gyrase intgeraction with coumarin-based inhibitors-the role of the hydroxyl benzoate isopentenyl moiety and the 5'-methyl group of the noviose. Biochemistry 2002, 41, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Hurry, R.G.; Cortz, C.; Ananthanaraxan, T.P.; Schmolka, S. A new coumarin synthesis and its utilization for the synthesis of polycyclic coumarin compounds with anticarcinogenic properties. J. Org. Chem. 1998, 53, 3936–3943. [Google Scholar]
- Hafez, E.A.A.; Elnagdi, M.H.; Elagamey, A.G.A.; EL-Taweel, F.M.A.A. Nitriles in Heterocyclic Synthesis: Novel Synthesis of Benzo[c]Coumarin and of Benzo[c]Pyrano[3,2-c]Quinoline Derivatives. Heterocycles 1987, 26, 903–907. [Google Scholar] [CrossRef]
- Tanabe, A.; Nakashima, H.; Yoshida, O.; Yamamoto, N.; Tenmyo, O.; Oki, T. Inhibitory Effect of New Antibiotic, Pradimicin A on Infectivity, Cytopathic Effect and Replication of Human Immunodeficiency Virus in Vitro. J. Antibiot. 1988, 41, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Shijay, G.; Cheng, H.T.; Chi, T.; Ching-Fa, Y. Fluoride Ion Catalyzed Multicomponet Reactions for Efficient Synthesis of 4H-Chromene and N-Arylquinoline Derivates in Aqueous Media. Tedrahedron 2008, 64, 9143–9149. [Google Scholar] [CrossRef]
- Balaji, P.N.; Lakshmi, L.K.; Mohan, K.; Revathi, K.; Chamundeswari, A.; Indrani, P.M. In-vitro anti-inflammatory and antimicrobial activity of synthesized some novel pyrazole derivatives from coumarin chalcones. Der Pharmacia Sinica 2012, 3, 685–689. [Google Scholar]
- Bayer, T.A.; Schafer, S.; Breyh, H.; Breyhan, O.; Wirths, C.; Treiber, G.A. A Vicious Circle: Role of Oxidative Stress, Intraneuronal Aβ and Cu in Alzheimer’s Disease Multhaup. Clin. Neuropathol. 2006, 25, 163–171. [Google Scholar] [PubMed]
- Fokialakis, N.; Magiatis, P.; Chinou, L.; Mitaka, S.; Tillequin, F.; Megistoquinones, I, II. Two Quinoline Alkaloids with Antibacterial Activity from the Bark of Sarcomelicope megistophylla. Chem. Pharm. Bull. 2002, 50, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Beagley, P.; Blackie, M.A.L.; Chibale, K.; Clarkson, C.; Meijboom, R.; Moss, J.R.; Smith, P.; Su, H. Synthesis and Antiplasmodial Activity in Vitro of New Ferrocene-Chloroquine Analogues. Dalton Trans. 2003, 3046–3051. [Google Scholar]
- Morgan, L.R.; Jursic, B.S.; Hooper, C.L.; Neumann, D.M.; Thangaraj, K.; Leblance, B. Anticancer Activity for 4,4-Dihydroxybenzophenone-2,4-Dinitrophenylhydrazone (A-007) Analogues and Their Abilities to interact with Lymphoendothelial Cell Surface Markers. Bioorg. Med. Chem. Lett. 2002, 12, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, L.; Loy, G.; Secci, D.; Calignano, A. Synthesis and Pharmacological Activity of 2-oxo-(2H) 1-Benzopyran-3-Carboxamide Derivatives. Eur. J. Med. Chem. 1993, 28, 517–520. [Google Scholar] [CrossRef]
- Cannon, J.G.; Khonji, R.R. Centrally Acting Emetics. 9. Hofmann and Emde Degradation Products of Nuciferine. J. Med. Chem. 1975, 18, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Blampain, G.; Blampain, P.; Georges, A.J.; Abessolo, H.; et al. Synthesis and Antimalarial Activity in Vitro and in Vivo of a New Ferrocene-Chloroquine Analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Galil, F.M.; Riad, B.Y.; Sherif, S.M.; Elnagdi, M.H. Activated Nitriles in Heterocyclic Synthesis: A Novel Synthesis of 4-Azoloyl-2- Aminoquinolines. Chem. Lett. 1982, 11, 1123–1126. [Google Scholar] [CrossRef]
- Shaker, R.M. Synthesis and Reactions of Some New 4H-Pyrano[3,2-c]benzopyran-5-One Derivatives and Their Potential Biological Activities. Pharmazie 1996, 51, 148–151. [Google Scholar] [PubMed]
- Balalaie, S.; Abdolmohammadi, S. Novel and Efficient Catalysts for the One-Pot Synthesis of 3,4-Dihydropyrano[c]Chromene Derivatives in Aqueous Media. Tetrahedron Lett. 2007, 48, 3299–3303. [Google Scholar] [CrossRef]
- Kidwai, M.; Saxena, S. Convenient Preparation of Pyrano Benzopyranes in Aqueous Media. Synth. Commun. 2006, 36, 2737–2742. [Google Scholar] [CrossRef]
- Heravi, M.M.; Alimadadi, J.B.; Derikvand, F.; Bamoharram, F.F.; Oskooie, H.A. Three Component, One-Pot Synthesis of Dihydropyrano[3,2-c]Chromene Derivatives in the Presence of H6P2W18O62·18H2O as a Green and Recyclable Catalyst. Catal. Commun. 2008, 10, 272–275. [Google Scholar] [CrossRef]
- Seifi, M.; Sheibani, H. High Surface Area MgO as a Highly Effective Heterogeneous Base Catalyst for Three-Component Synthesis of Tetra hydro benzopyran and 3,4-Dihydropyrano[c]chromene Derivatives in Aqueous Media. Catal. Lett. 2008, 126, 275–279. [Google Scholar] [CrossRef]
- Khurana, J.M.; Kumar, S. Tetra Buthyl Ammonium Bromide (TBAB): A Neutral and Efficient Catalyst for the Synthesis of Biscoumarin and 3,4-Dihydopyrano[c]Chromene Derivatives in Water and Solvent-Free Conditions. Tetrahedron Lett. 2009, 50, 4125–4127. [Google Scholar] [CrossRef]
- Dobaria, A.V.; Patel, J.R.; Padaliya, J.V.; Parekh, H.H. Thiazolidinones bearing chloroquinoline nucleus as potential antimicrobial agents. Indian J. Heterocycl. Chem. 2001, 11, 115–118. [Google Scholar]
- Deepak, K.; Bux, F.B.; Varsh, P.; Arun, S. Thiazolidinone: Synthesis and biological studies. Der Pharma Chemica 2012, 4, 538–543. [Google Scholar]
- Shweta, T.D.; Pratima, R.P.S.; Toraskar, M.P. Synthesis and antimicrobial evaluation of some 4-substituted thiazolidinone derivatives. Der Pharma Chemica 2010, 2, 17–20. [Google Scholar]
- Raghav, M.; Isha, T.; Sachin, S.; Jha, K.K. Facile synthesis of thiazolidinones bearing thiophene nucleus as antimicrobial agents. Der Pharma Chemica 2012, 4, 489–496. [Google Scholar]
- Aamer, S.; Naeem, A.; Ulrich, F. Synthesis and Antibacterial Activity of some Novel 2-Aroylimino-3-aryl-thiazolidin-4-ones. J. Braz. Chem. Soc. 2007, 18, 559–565. [Google Scholar] [CrossRef]
- El Azab, I.H.; El Rady, E.A. Facile and Simple Synthesis of some New Polyfunctionally Heterocyclic Derivatives: Incorporating 2-Imino-2H-Chromene Moiety. J. Heterocycl. Chem. 2012, 49, 135–148. [Google Scholar] [CrossRef]
- El Rady, E.A.; El Azab, I.H. Reactivity of β-enamino ester of benzo[f]chromene: One pot synthesis of isolated and fused heterocyclic derivatives of benzo[f]chromene. Eur. J. Chem. 2012, 3, 81–86. [Google Scholar] [CrossRef]
- El Azab, I.H. Synthesis of Some New Benzo[b][1,4]diazepine Based Heterocycles. J. Heterocycl. Chem. 2013, 50, 178–188. [Google Scholar] [CrossRef]
- Azab, I.H.; kenzy, N.A.A. Synthesis of Fused- Isolated Azoles and N-Heteroaryl Derivatives Based on 2-Methyl-3,4-dihydrothieno[3,4-d]pyrimidin-5-amine. Synth. Commun. 2014, 44, 2692–2714. [Google Scholar] [CrossRef]
- El Azab, I.H.; El Rady, E.A. Simple Method for Synthesis of Isolated Heterocyclic Compounds: Incorporating 2-(2-Bromoacetyl)-isoindoline-1,3-dione and 2-(2-Cyanoacetyl)isoindo-line-1,3-dione. Indian J. Chem. 2014, 52, 1194–1204. [Google Scholar]
- Pal, R.; Handa, R.N.; Pujari, H.K. Heterocyclic systems containing bridgehead nitrogen atom: Part LXIII. Reaction of 1-Methyl-7,8,10,11-tetraazaspiro[5,5]undecane-9-thione with bi functional compounds. Indian J. Chem. Soc. 1992, 31B, 771–777. [Google Scholar]
- Cappuccino, J.G.; Sherman, N. Microbiology- A Laboratory Manual, 4th ed.; Addison-Wesley, Longman, Inc.: Ithaca, NY, USA, 1999; pp. 477–487. [Google Scholar]
- Sample Availability: Samples of the compounds 4, 5, 11, 16, 17 and 20 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Azab, I.H.; Youssef, M.M.; Amin, M.A. Microwave-Assisted Synthesis of Novel 2H-Chromene Derivatives Bearing Phenylthiazolidinones and Their Biological Activity Assessment. Molecules 2014, 19, 19648-19664. https://doi.org/10.3390/molecules191219648
El Azab IH, Youssef MM, Amin MA. Microwave-Assisted Synthesis of Novel 2H-Chromene Derivatives Bearing Phenylthiazolidinones and Their Biological Activity Assessment. Molecules. 2014; 19(12):19648-19664. https://doi.org/10.3390/molecules191219648
Chicago/Turabian StyleEl Azab, Islam H., Mohamed M. Youssef, and Mahmoud A. Amin. 2014. "Microwave-Assisted Synthesis of Novel 2H-Chromene Derivatives Bearing Phenylthiazolidinones and Their Biological Activity Assessment" Molecules 19, no. 12: 19648-19664. https://doi.org/10.3390/molecules191219648