Formation of Combustible Hydrocarbons and H2 during Photocatalytic Decomposition of Various Organic Compounds under Aerated and Deaerated Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Decomposition of Various Organic Compounds: The Influence of a Substrate on the Formation of the Gaseous and Liquid Products
| Substrate | Composition of a Gas Phase | Composition of a Liquid Phase |
|---|---|---|
| CH3COOH | CH4, CO2, C2H6, C3H8, H2 | CH3COOH, CH3OH, C2H5OH, CO(CH3)2, CH3CHO, CH3COOCH3 |
| CH3OH | CH4 a, CO2, H2 | CH3OH, CH3CHO |
| C2H5OH | CH4, CO2, C2H6, H2 | C2H5OH, CH3CHO, CH3OH |
| C6H12O6 | CH4, CO2, H2 | C6H12O6, CH3CHO, C2H5OH, CH3COOCH3 |
2.2. Effect of the Reaction Atmosphere on Gas Phase Composition during the Photodegradation of Various Organic Substrates
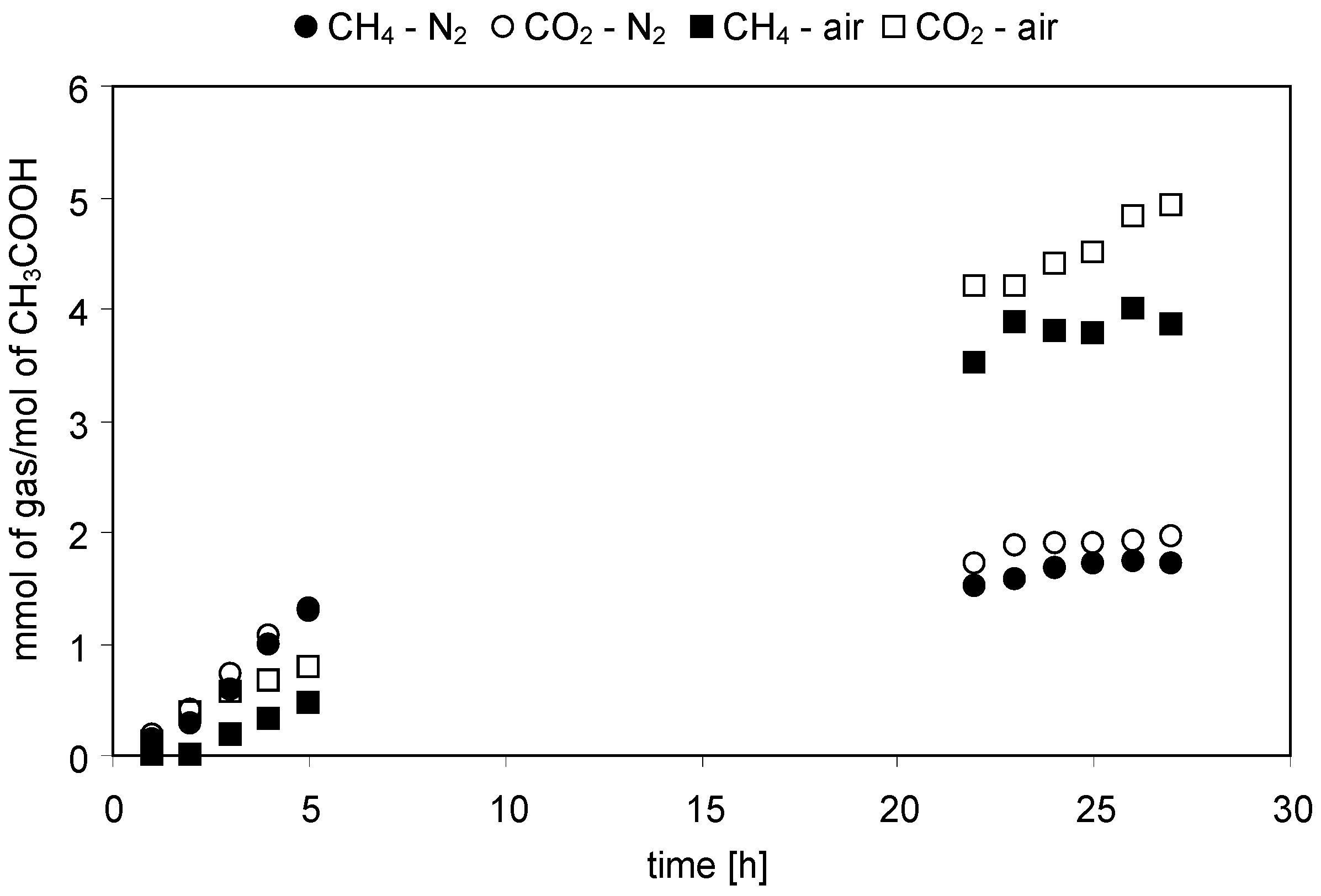
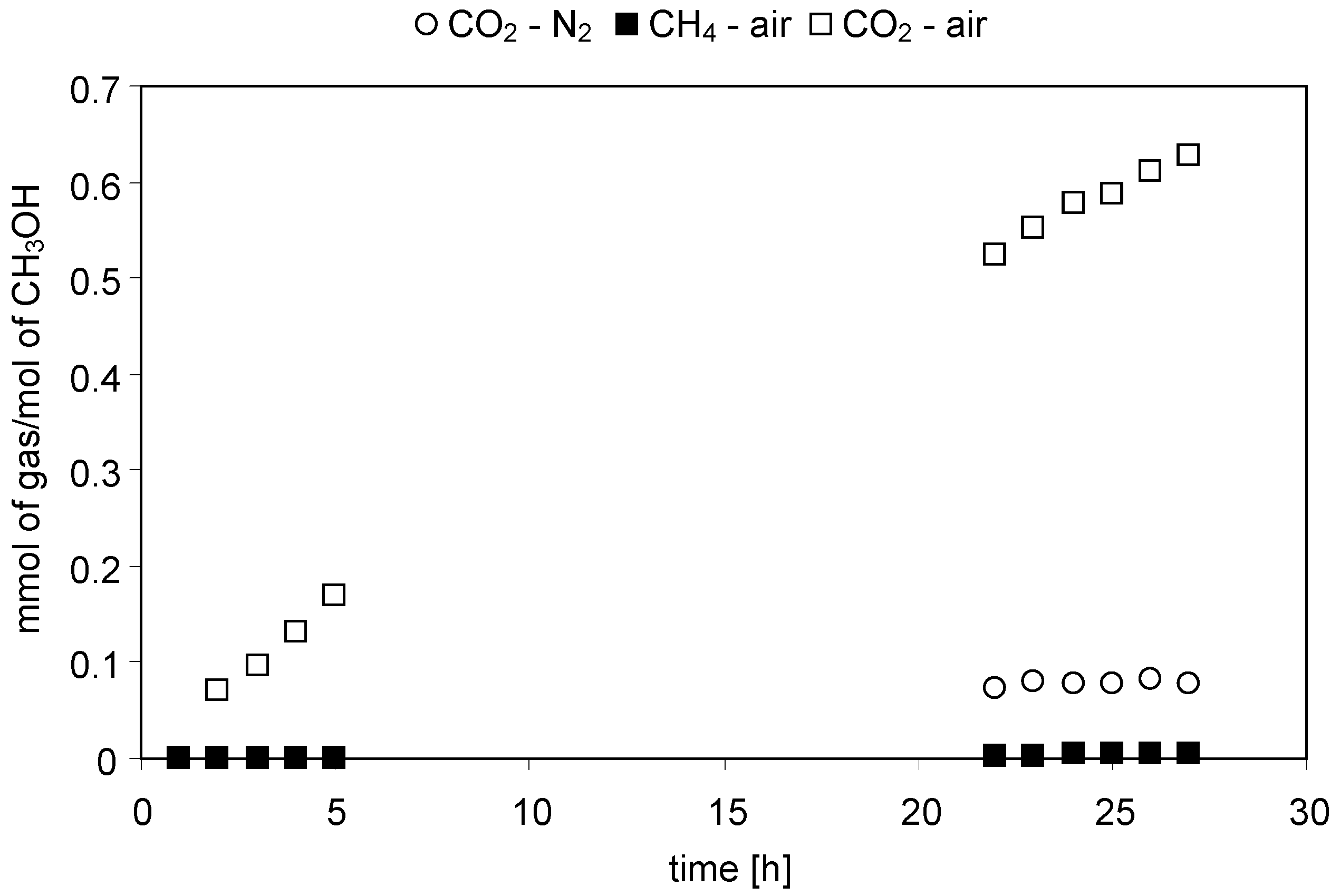
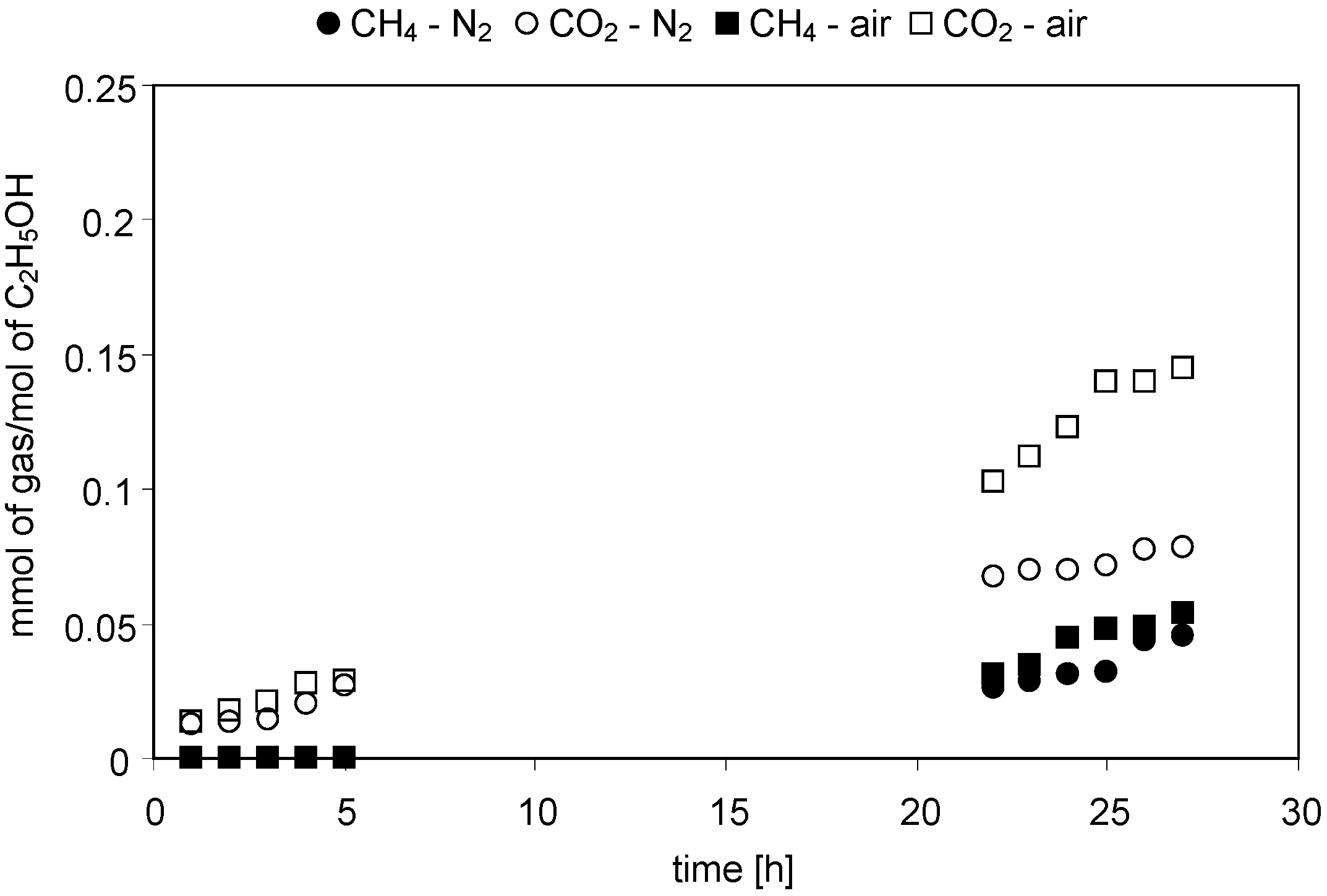
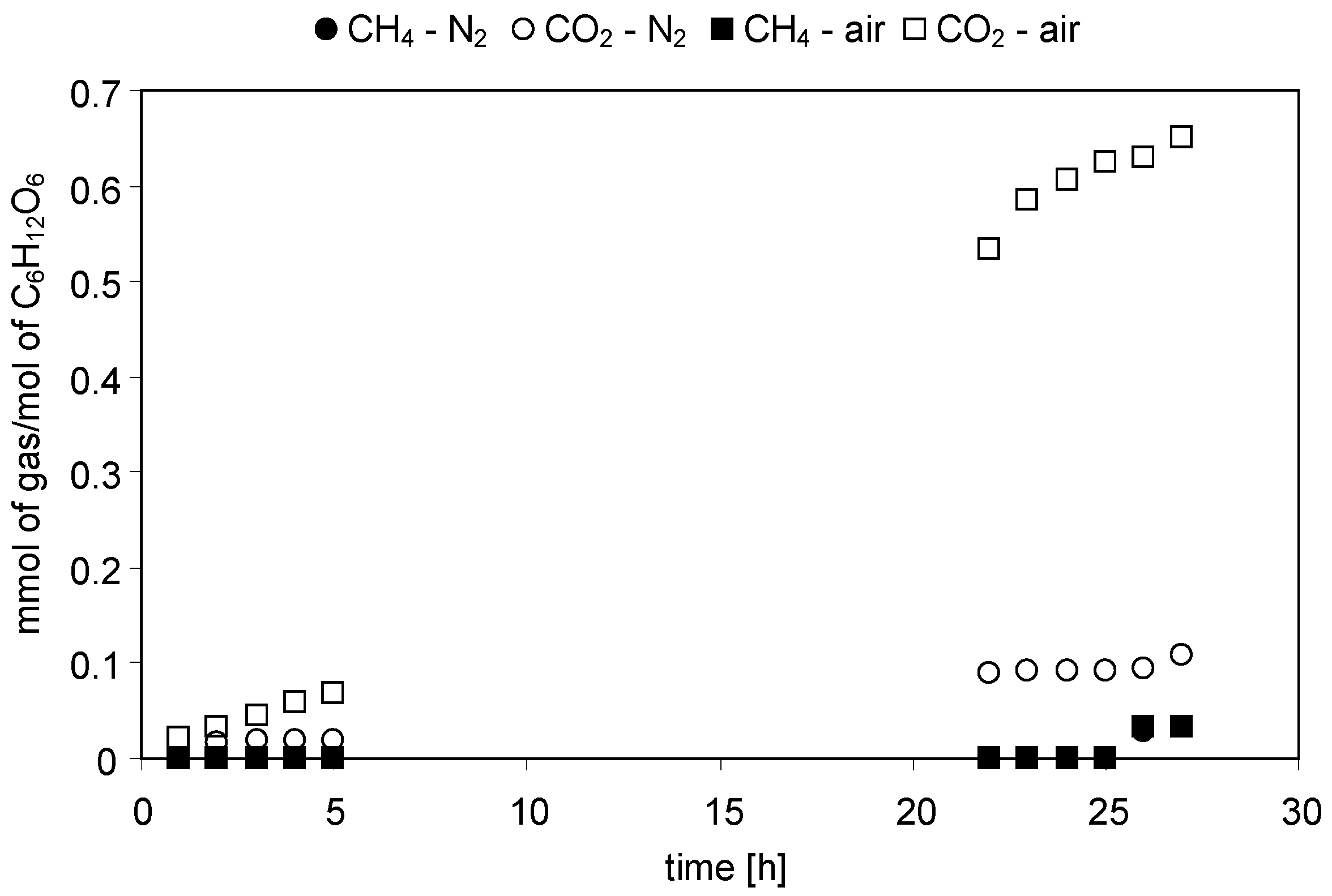
2.2.1. Acetic Acid
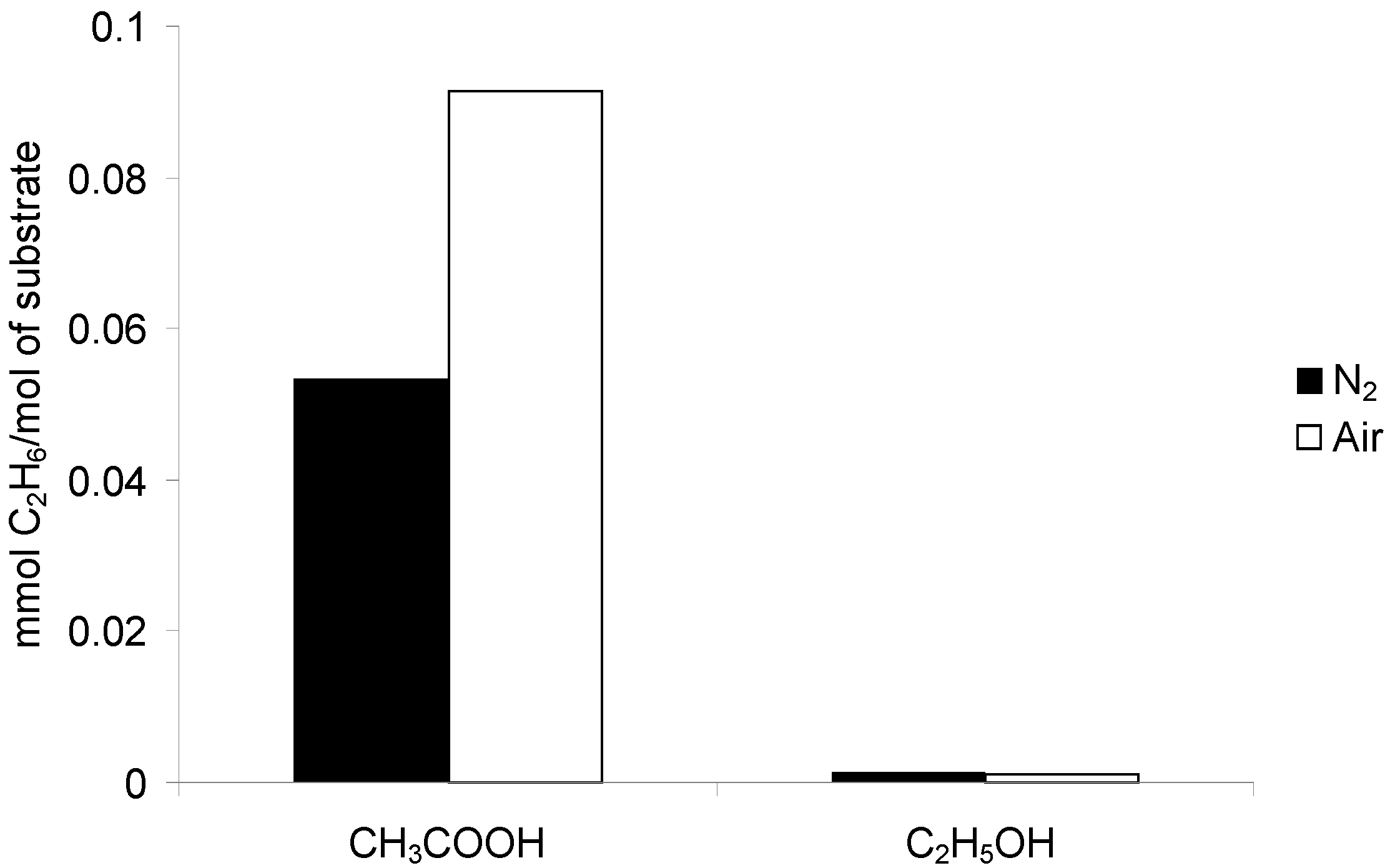
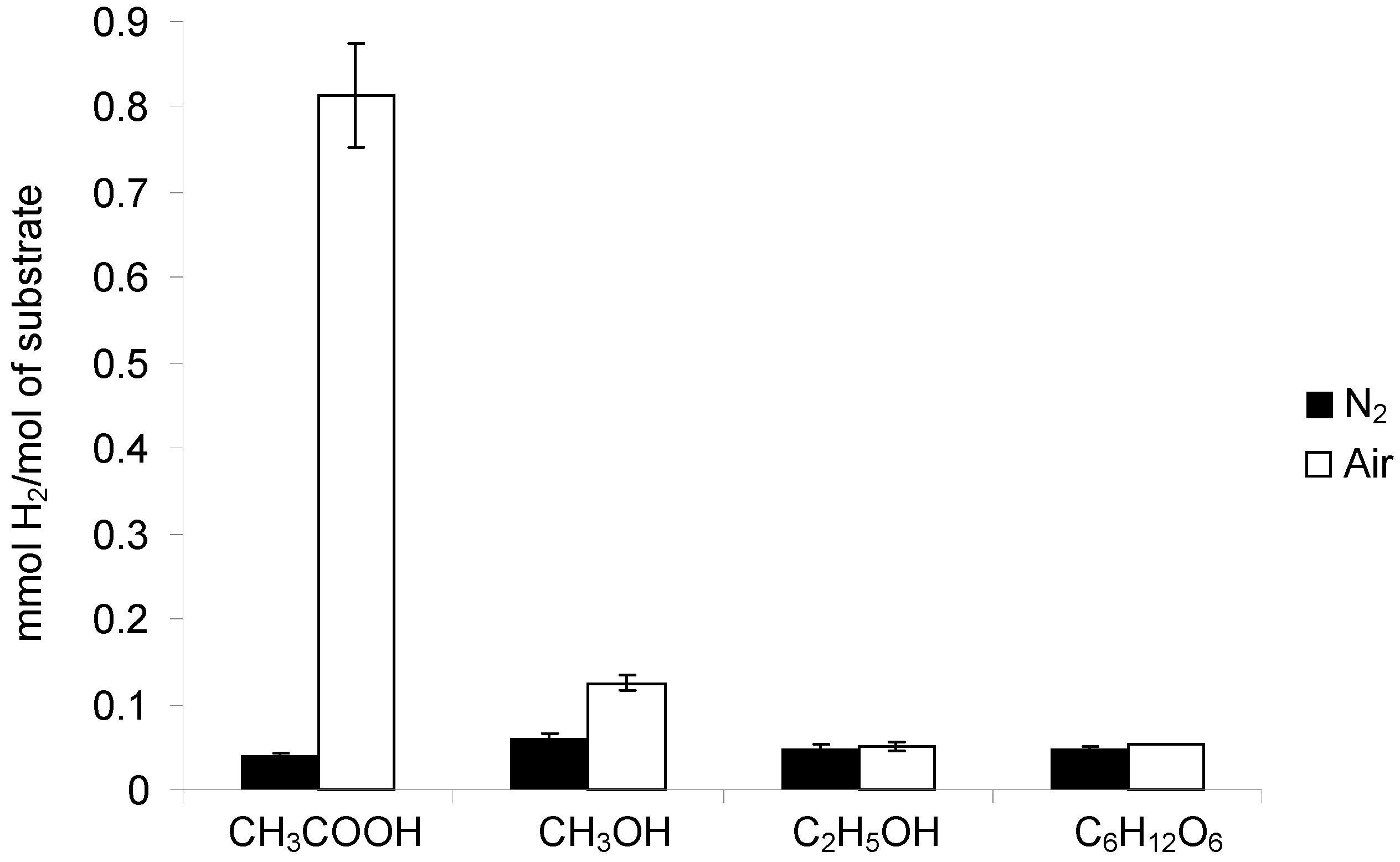
2.2.2. Methanol
2.2.3. Ethanol
2.2.4. Glucose
2.3. Hydrogen Evolution in the Presence of Oxygen: a Point of Discussion
3. Experimental Section
3.1. Photocatalyst
3.2. Photocatalytic Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirel, B.; Scherer, P. Bio-methanization of energy crops through mono-digestion for continuous production of renewable biogas. Renew. Energy 2009, 34, 2940–2945. [Google Scholar]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Hanaki, K.; Hirunmasuwan, S.; Matsuo, T. Protection of methanogenic bacteria from low pH and toxic materials by immobilization using polyvinyl alcohol. Water Res. 1994, 28, 877–885. [Google Scholar] [CrossRef]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance. J. Photochem. Photobiol. C 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Tan, S.S.; Zou, L.; Hu, E. Photosynthesis of hydrogen and methane as key components for clean energy system. Sci. Technol. Adv. Mater. 2007, 8, 89–92. [Google Scholar] [CrossRef]
- Subrahmanyam, M.; Kaneco, S.; Alonso-Vante, N. A screening for the photo reduction of carbon dioxide supported on metal oxide catalysts for C1–C3 selectivity. Appl. Catal. B 1990, 23, 169–174. [Google Scholar] [CrossRef]
- Asi, M.A.; He, C.; Su, M.; Xia, D.; Lin, L.; Deng, H. Photocatalytic reduction of CO2 to hydrocarbons using AgBr/TiO2 nanocomposites under visible light. Catal. Today 2011, 175, 256–263. [Google Scholar] [CrossRef]
- Collado, L.; Jana, P.; Sierra, B.; Coronado, J.M.; Pizarro, P.; Serrano, D.P.; de la Peña O’Shea, V.A. Enhancement of hydrocarbon production via artificial photosynthesis due to synergetic effect of Ag supported on TiO2 and ZnO semiconductors. Chem. Eng. J. 2013, 224, 128–135. [Google Scholar] [CrossRef]
- Tahir, M.; Saidina Amin, N. Recycling of carbon dioxide to renewable fuels by photocatalysis: Prospects and challenges. Renew. Sustain. Energy Rev. 2013, 25, 560–579. [Google Scholar] [CrossRef]
- Mei, B.; Pougin, A.; Strunk, J. Influence of photodeposited gold nanoparticles on the photocatalytic activity of titanate species in the reduction of CO2 to hydrocarbons. J. Catal. 2013, 306, 184–189. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, B.; Bard, A.J. Photoelectrosynthesis of ethane from acetate ion at an n-type TiO2 electrode. The photo-Kolbe reaction. J. Am. Chem. Soc. 1977, 99, 7729–7731. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic synthesis of methane from acetic acid–new Kolbe reaction pathway. J. Am. Chem. Soc. 1978, 100, 2239–2240. [Google Scholar] [CrossRef]
- Kraeutler, B.; Jaeger, C.D.; Bard, A.J. Direct observation of radical intermediates in the photo–Kolbe reaction–Heterogeneous photocatalytic radical formation by electron spin resonance. J. Am. Chem. Soc. 1978, 100, 4903–4905. [Google Scholar]
- Sakata, T.; Kawai, T.; Hashimoto, K. Heterogeneous photocatalytic reactions of organic acids in water. New reaction paths besides the photo-Kolbe reaction. J. Phys. Chem. 1984, 88, 2344–2350. [Google Scholar] [CrossRef]
- Dey, G.R.; Pushpa, K.K. Formation of different products during photo-catalytic reaction on TiO2 suspension in water with and without 2-propanol under diverse ambient conditions. Res. Chem. Intermed. 2006, 32, 725–736. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Saeed Al-Mazroai, L.; Dickinson, A.; Greaves, J.; James, D.; Millard, L.; Pedrono, F. Sustainable H2 gas production by photocatalysis. J. Photochem. Photobiol. A 2010, 216, 115–118. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, Y.; Zhang, J.; Wang, X.; Feng, Z.; Li, C. Enhancing hydrogen production activity and suppressing CO formation from photocatalytic biomass reforming on Pt/TiO2 by optimizing anatase–rutile phase structure. J. Catal. 2011, 278, 329–335. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrog. Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Klauson, D.; Budarnaja, O.; Beltran, I.C.; Krichevskaya, M.; Preis, S. Photocatalytic decomposition of humic acids in anoxic aqueous solutions producing hydrogen, oxygen and light hydrocarbons. Environ. Technol. 2014, 35, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S.; Heciak, A.; Morawski, A.W. Photocatalytic acetic acid decomposition leading to the production of hydrocarbons and hydrogen on Fe-modified TiO2. Catal. Today 2011, 161, 189–195. [Google Scholar] [CrossRef]
- Asal, S.; Saif, M.; Hafez, H.; Mozia, S.; Heciak, A.; Moszyński, D.; Abdel-Mottaleb, M.S.A. Photocatalytic generation of useful hydrocarbons and hydrogen from acetic acid in the presence of lanthanide modified TiO2. Int. J. Hydrog. Energy 2011, 36, 6529–6537. [Google Scholar]
- Muggli, D.; Falconer, J.L. Parallel pathways for hotocatalytic decomposition of acetic acid on TiO2. J. Catal. 1999, 197, 230–237. [Google Scholar] [CrossRef]
- Blount, M.C.; Buchholz, J.A.; Falconer, J.L. Photocatalytic decomposition of aliphatic alcohols, acids, and esters. J. Catal. 2001, 197, 303–314. [Google Scholar]
- Chen, J.; Ollis, D.F.; Rulkens, W.H.; Bruning, H. Photocatalyzed oxidation of alcohols and organochlorides in the presence of native TiO2 and metallized TiO2 suspensions. Part (II): Photocatalytic mechanisms. Water Res. 1999, 33, 669–676. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Peng, S.; Lu, G.; Li, S. Photocatalytic hydrogen generation in the presence of glucose over ZnS-coated ZnIn2S4 under visible light irradiation. Int. J. Hydrog. Energy 2010, 35, 7116–7126. [Google Scholar] [CrossRef]
- Dey, G.R.; Nair, K.N.R.; Pushpa, K.K. Photolysis studies on HCOOH and HCOO− in presence of TiO2 photocatalyst as suspension in aqueous medium. J. Nat. Gas Chem. 2009, 18, 50–54. [Google Scholar] [CrossRef]
- Korzhak, A.V.; Kuchmii, S.Y.; Kryukow, A.I. Effects of activation and inhibition by oxygen of the photocatalytic evolution of hydrogen from alcohol-water media. Theor. Exp. Chem. 1994, 30, 26–29. [Google Scholar] [CrossRef]
- Borgarello, E.; Serpone, N.; Pelizzetti, E.; Barbeni, M. Efficient photochemical conversion of aqueous sulphides and sulphites to hydrogen using a rhodium-loaded CdS photocatalyst. J. Photochem. 1986, 33, 35–48. [Google Scholar] [CrossRef]
- Medrano, J.A.; Oliva, A.; Ruiz, J.; Garcia, L.; Arauzo, J. Catalytic steam reforming of acetic acid in a fluidized bed reactor with oxygen addition. Int. J. Hydrog. Energy 2008, 33, 4387–4396. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Leung, D.Y.C.; Xue, W.; Ding, Z. Photocatalytic reforming of glucose over La doped alkali tantalate photocatalysts for H2 production. Catal. Commun. 2010, 12, 184–187. [Google Scholar] [CrossRef]
- Rosseler, O.; Shankar, M.V.; Karkmaz-Le Du, M.; Schmidlin, L.; Keller, N.; Keller, V. Solar light photocatalytic hydrogen production from water over Pt and Au/TiO2(anatase/rutile) photocatalysts: Influence of noble metal and porogen promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Strataki, N.; Bekiari, V.; Kondarides, D.I.; Lianos, P. Hydrogen production by photocatalytic alcohol reforming employing highly efficient nanocrystalline titania films. Appl. Catal. B 2007, 77, 184–189. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Su, W.; Zhou, G.; Zong, X.; Lei, Z.; Li, C. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrog. Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Fu, X.; Leung, D.Y.C.; Wang, X.; Xue, W.; Fu, X. Photocatalytic reforming of ethanol to H2 and CH4 over ZnSn(OH)6 nanocubes. Int. J. Hydrog. Energy 2011, 36, 1524–1530. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozia, S.; Kułagowska, A.; Morawski, A.W. Formation of Combustible Hydrocarbons and H2 during Photocatalytic Decomposition of Various Organic Compounds under Aerated and Deaerated Conditions. Molecules 2014, 19, 19633-19647. https://doi.org/10.3390/molecules191219633
Mozia S, Kułagowska A, Morawski AW. Formation of Combustible Hydrocarbons and H2 during Photocatalytic Decomposition of Various Organic Compounds under Aerated and Deaerated Conditions. Molecules. 2014; 19(12):19633-19647. https://doi.org/10.3390/molecules191219633
Chicago/Turabian StyleMozia, Sylwia, Aleksandra Kułagowska, and Antoni W. Morawski. 2014. "Formation of Combustible Hydrocarbons and H2 during Photocatalytic Decomposition of Various Organic Compounds under Aerated and Deaerated Conditions" Molecules 19, no. 12: 19633-19647. https://doi.org/10.3390/molecules191219633




