Influence of Post-Treatment Operations on Structural Properties and Photocatalytic Activity of Octahedral Anatase Titania Particles Prepared by an Ultrasonication-Hydrothermal Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Original OAP-Containing Sample
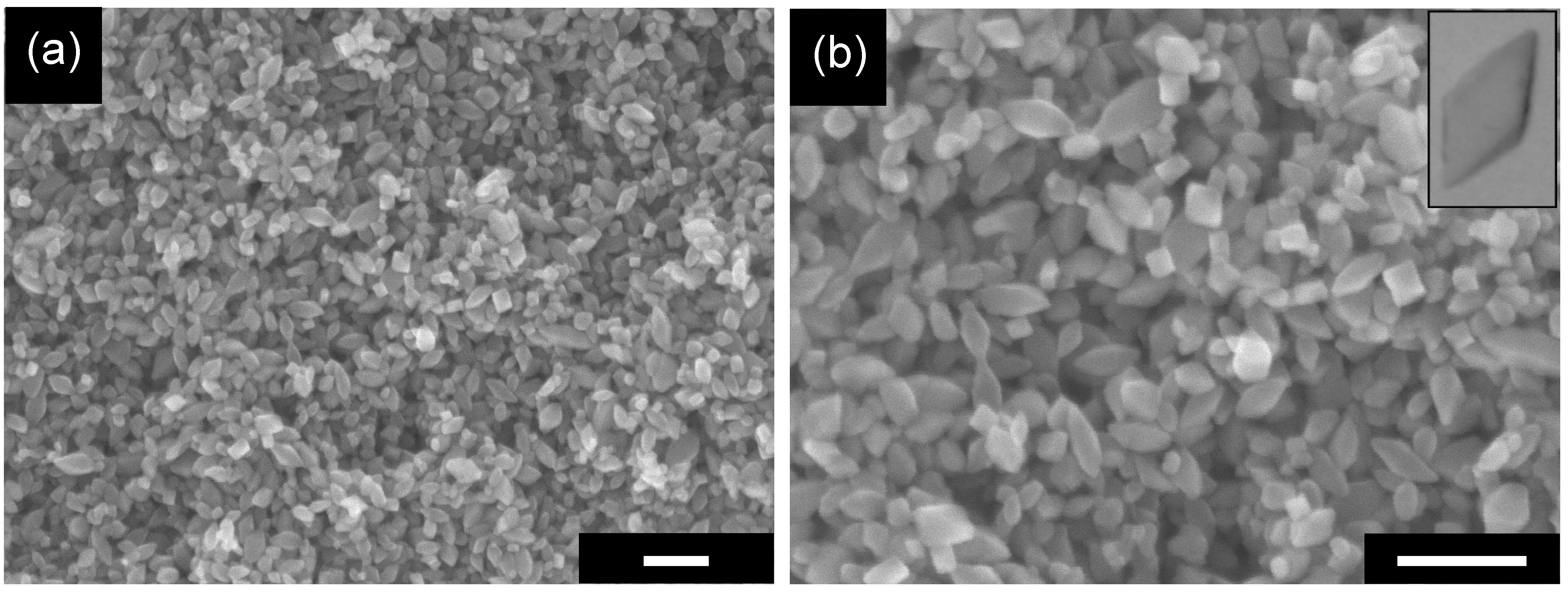
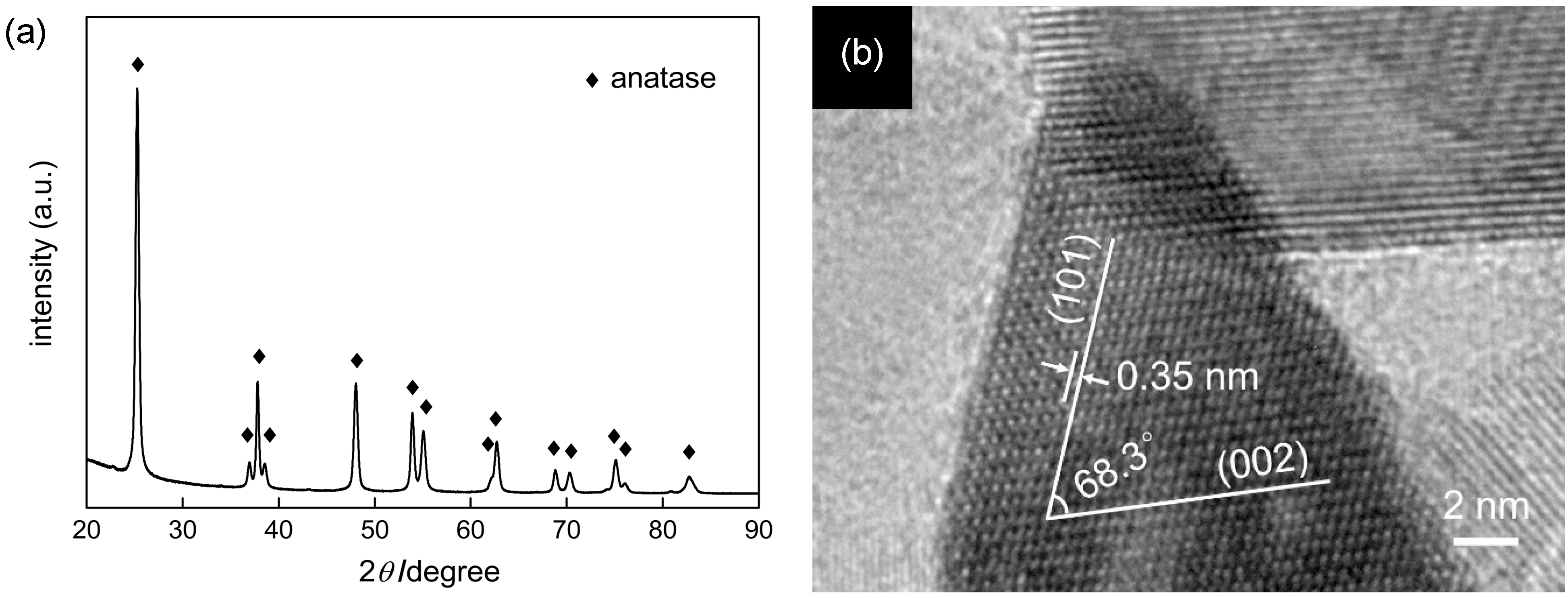
2.2. Influence of Calcination on the Structure of Particles

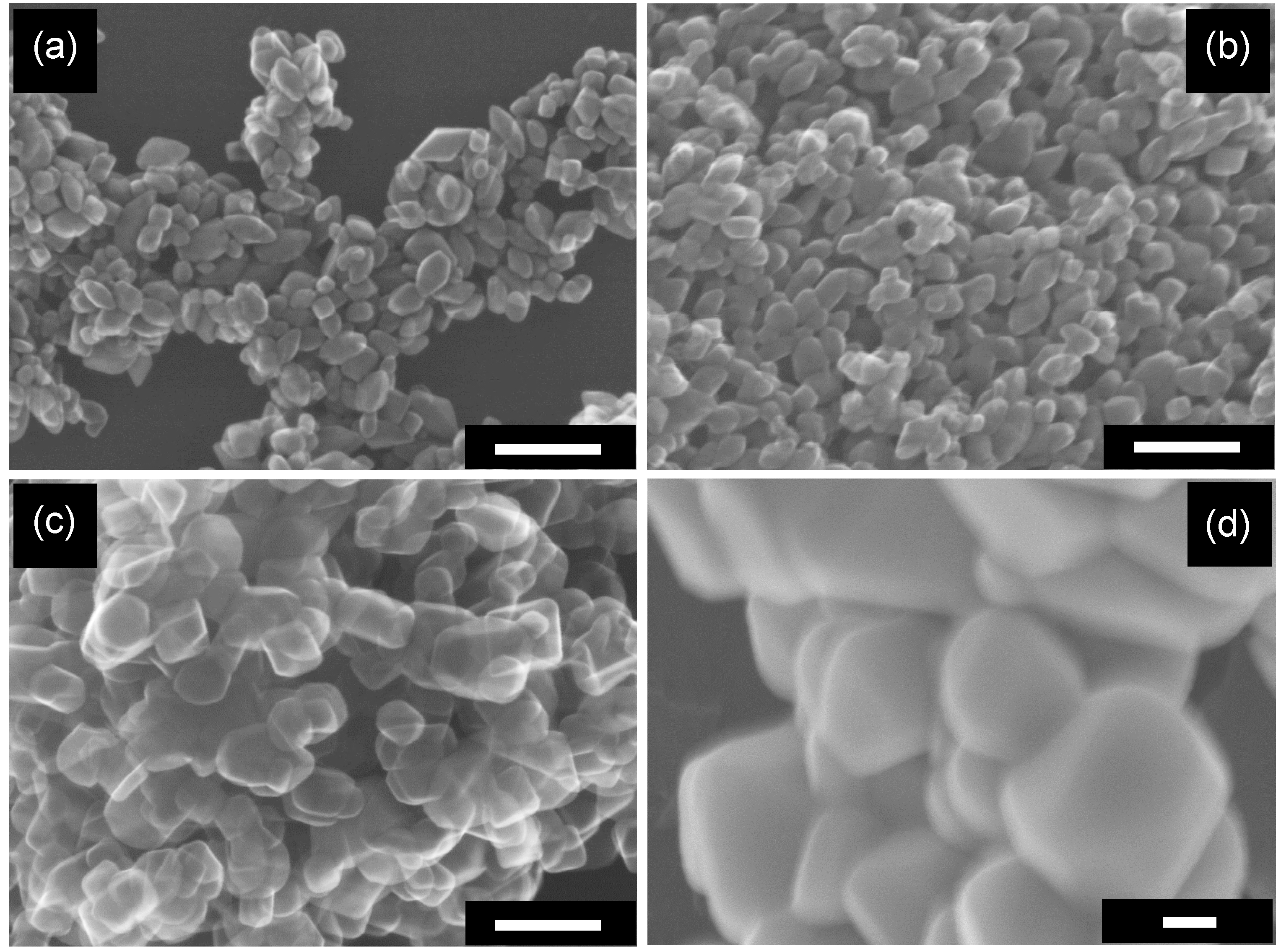
2.3. Influence of Calcination on Photocatalytic Activities
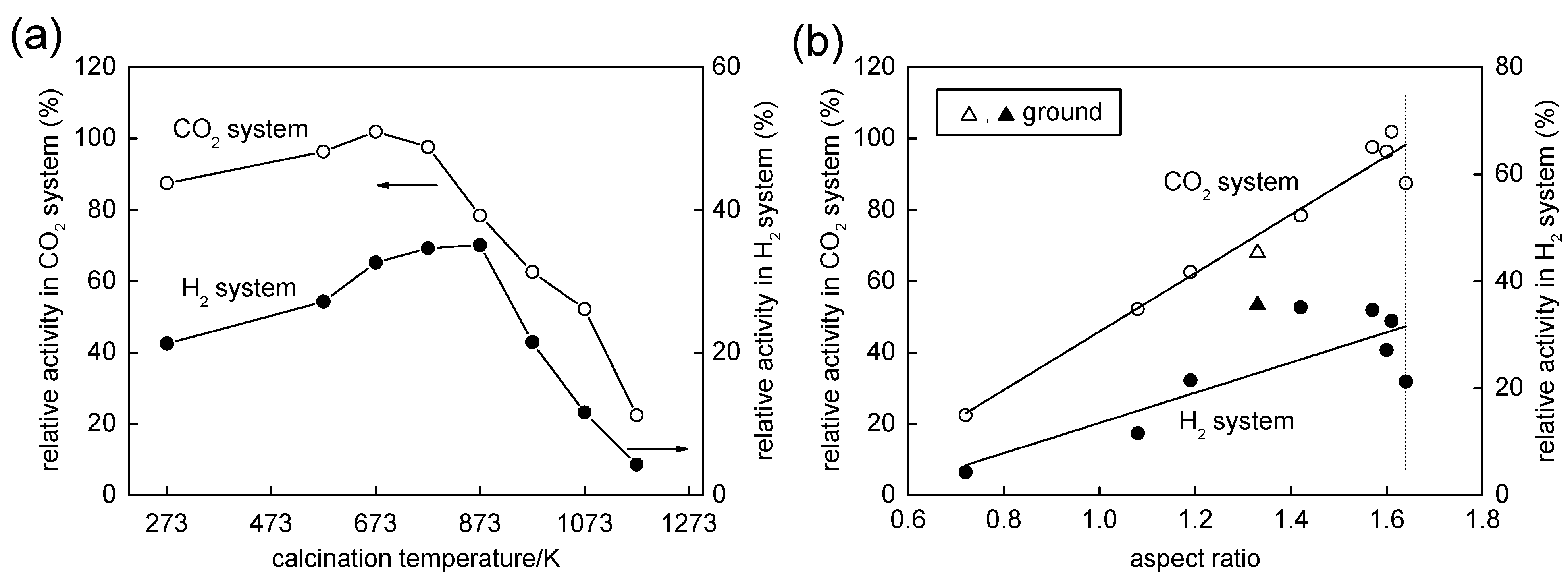
2.4. Time-Resolved Microwave Conductivity
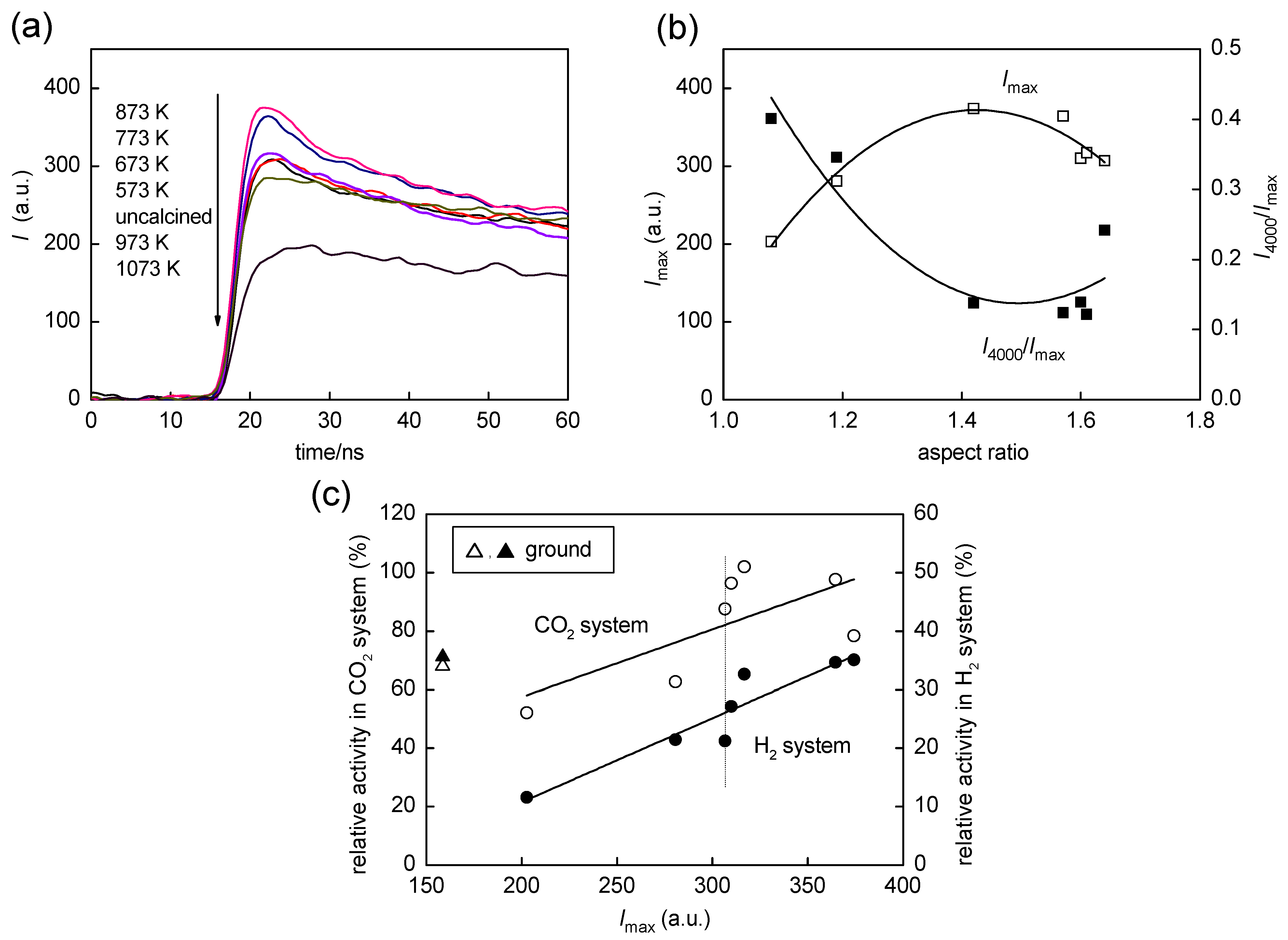
2.5. Influence of Grinding on Structure and Photocatalytic Activity of OAP-Containing Particles
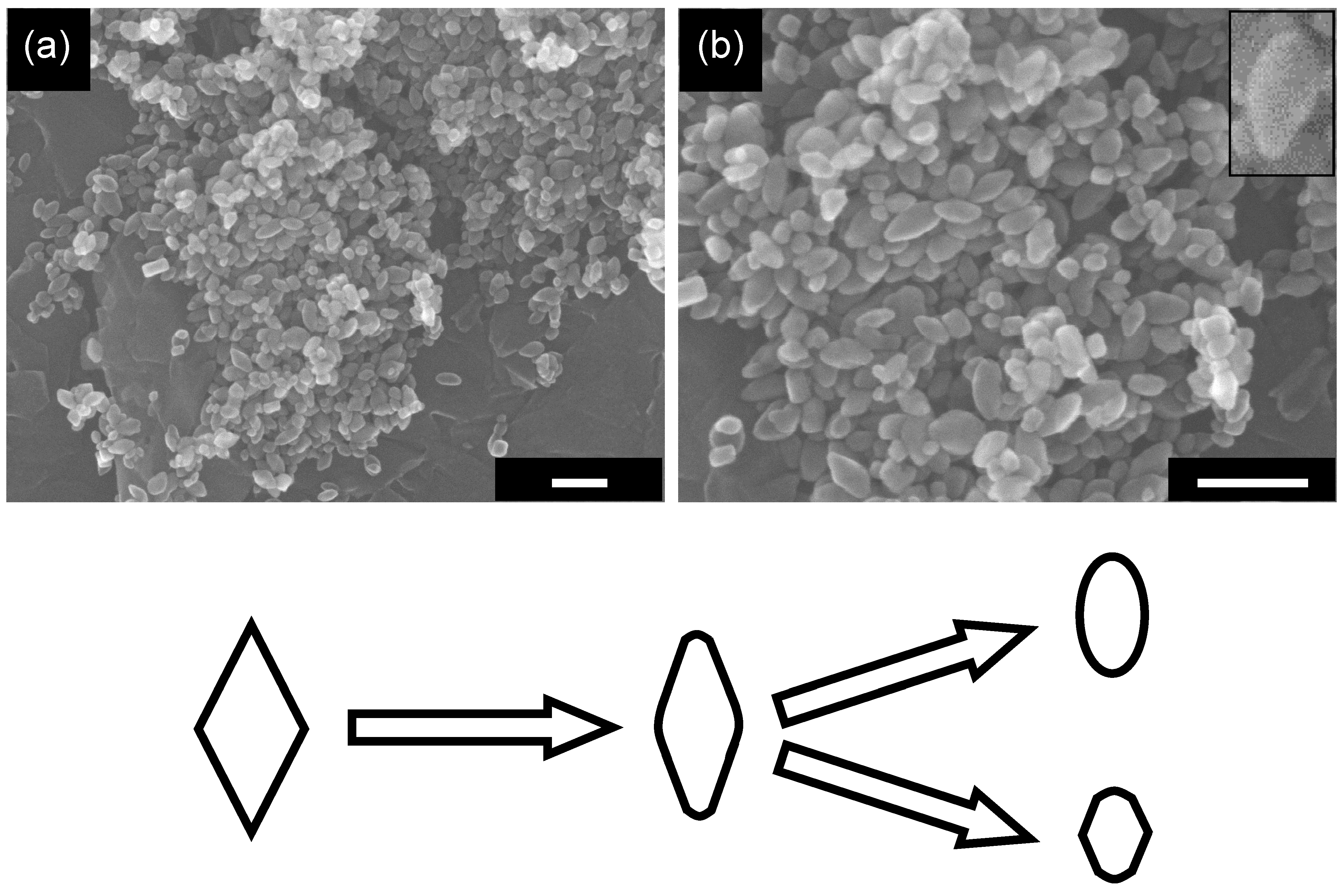
3. Experimental Section
3.1. Preparation of OAPs Samples
3.2. Characterization
3.3. Photocatalytic Activity Test
3.4. Time-Resolved Microwave Conductivity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar]
- Ohtani, B. Preparing articles on photocatalysis—Beyond the illusions, misconceptions and speculation. Chem. Lett. 2008, 37, 217–229. [Google Scholar]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C 2010, 11, 157–178. [Google Scholar]
- Ohtani, B. Revisiting the fundamental physical chemistry in heterogeneous photocatalysis: Its thermodynamics and kinetics. Phys. Chem. Chem. Phys. 2014, 16, 1788–1797. [Google Scholar]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar]
- Zhang, H.M.; Han, Y.H.; Liu, X.L.; Liu, P.R.; Yu, H.; Zhang, S.Q.; Yao, X.D.; Zhao, H.J. Anatase TiO2 microspheres with exposed mirror-like plane {001} facets for high performance dye-sensitized solar cells (DSSCs). Chem. Commun. 2010, 46, 8395–8397. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2010, 50, 2904–2939. [Google Scholar]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar]
- Jun, J.W.; Casula, M.F.; Sim, J.H.; Kim, S.Y.; Cheon, J.; Alivisatos, A.P. Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 2003, 125, 15981–15985. [Google Scholar]
- Barnard, A.S.; Curtiss, L.A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 2005, 5, 1261–1266. [Google Scholar]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-controlled anatase titanium(IV) oxide particles prepared by hydrothermal treatment of peroxo titanic acid in the presence of polyvinyl alcohol. J. Phys. Chem. C 2007, 113, 3062–3069. [Google Scholar]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar]
- Liu, S.; Yu, J.; Wang, W. Effects of annealing on the microstructures and photoactivity of fluorinated N-doped TiO2. Phys. Chem. Chem. Phys. 2010, 12, 12308–12315. [Google Scholar]
- Wei, Z.; Liu, Y.; Wang, H.; Mei, Z.; Ye, J.; Wen, X.; Gu, L.; Xie, Y. A gas-solid reaction growth of dense TiO2 nanowire arrays on Ti foils at ambient atmosphere. J. Nanosci. Nanotechnol. 2012, 12, 316–323. [Google Scholar]
- Zaban, A.; Aruna, S.T.; Tirosh, S.; Gregg, B.A.; Mastai, Y. The effect of the preparation condition of TiO2 colloids on their surface structures. J. Phys. Chem. B 2000, 104, 4130–4133. [Google Scholar]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–642. [Google Scholar]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–260. [Google Scholar]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide. Chem. Commun. 2009, 17, 2311–2313. [Google Scholar]
- Ohtani, B.; Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Terada, Y. Highly active titania photocatalyst particles of controlled crystal phase, size, and polyhedral shape. Top. Catal. 2010, 53, 455–461. [Google Scholar]
- Wei, Z.; Kowalska, E.; Ohtani, B. Enhanced photocatalytic activity by particle morphology—Preparation, characterization and photocatalytic activities of octahedral anatase titania particles. Chem. Lett. 2014, 43, 346–348. [Google Scholar]
- Li, W.; Ni, C.; Lin, H.; Huang, C.P.; Ismat-Shah, S. Size dependence of thermal stability of TiO2 nanoparticles. J. Appl. Phys. 2004, 96, 6663–6668. [Google Scholar]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar]
- Kominami, H.; Murakami, S.; Kera, Y.; Ohtani, B. Titanium(IV) oxide photocatalyst of ultra-high activity: A new preparation process allowing compatibility of high adsorptivity and low electron-hole recombination probability. Chem. Lett. 1998, 125–125. [Google Scholar]
- Kominami, H.; Kumamoto, H.; Kera, Y.; Ohtani, B. Photocatalytic decolorization and mineralization of malachite green in an aqueous suspension of titanium(IV) oxide nano-particles under aerated conditions: correlation between some physical properties and their photocatalytic activity. J. Photochem. Photobiol. A: Chem. 2003, 160, 99–104. [Google Scholar]
- Grover, I.S.; Singh, S.; Pal, B. Stable anatase TiO2 formed by calcination of rice-like titania nanorod at 800 °C exhibits high photocatalytic activity. RSC Adv. 2014, 4, 24704–24709. [Google Scholar]
- Baiju, K.V.; Sibu, C.P.; Rajesh, K.; Krishna Pillaia, P.; Mukundan, P.; Warrier, K.G.K.; Wunderlich, W. An aqueous sol–gel route to synthesize nanosized lanthana-doped titania having an increased anatase phase stability for photocatalytic application. Mater. Chem. Phys. 2005, 90, 123–127. [Google Scholar]
- Wei, Z.; Kowalska, E.; Nitta, A.; Ohtani, B. Highly heat tolerant octahedral anatase particles—Effect of surface structure on the phase anatase-rutile transformation and photocatalytic activity. J. Mater. Chem. A 2014. in preparation. [Google Scholar]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Synthesis and characterization of decahedral-shape anatase titania photocatalyst particles in a coaxial flow gas-phase reaction. Chem. Mater. 2014. in preparation. [Google Scholar]
- Kominami, H.; Matsuura, T.; Iwai, K.; Ohtani, B.; Nishimoto, S.I.; Kera, Y. Ultra-highly active titanium(IV) oxide photocatalyst prepared by hydrothermal crystallization from titanium(IV) alkoxide in organic solvents. Chem. Lett. 1995, 693–694. [Google Scholar]
- Park, H.; Choi, W. Effects of TiO2 surface fluorination on photocatalytic reactions and photoelectrochemical behaviors. J. Phys. Chem. B 2004, 108, 4086–4093. [Google Scholar]
- Katoh, R.; Huijser, A.; Hara, K.; Savenije, T.J.; Siebbeles, L.D. A. Effect of the particle size on the electron injection efficiency in dye-sensitized nanocrystalline TiO2 films studied by time-resolved microwave conductivity (TRMC) measurements. J. Phys. Chem. C 2007, 111, 10741–10746. [Google Scholar]
- Colombo, D.P., Jr.; Bowman, R.M. Does interfacial charge transfer compete with charge carrier Recombination? A femtosecond diffuse reflectance investigation of TiO2 nanoparticles. J. Phys. Chem. 1996, 100, 18445–18449. [Google Scholar]
- Boettger, H.; Bryksin, V.V. Hopping conductivity in ordered and disordered solids (I). Phys. Status Solidi B 1976, 78, 9–56. [Google Scholar]
- Katoh, R.; Furube, A.; Yamanaka, K.; Morikawa, T. Charge separation and trapping in N-doped TiO2 photocatalysts: A time-resolved microwave conductivity study. J. Phys. Chem. C 2010, 1, 3261–3265. [Google Scholar]
- Ohtani, B.; Bowman, R.M.; Colombo, D.P.; Kominami, H.; Noguchi, H.; Uosaki, K. Femtosecond diffuse reflectance spectroscopy of aqueous titanium(IV) oxide suspension: Correlation of electron-hole recombination kinetics with photocatalytic activity. Chem. Lett. 1998, 579–580. [Google Scholar]
- Ohtani, B.; Majima, T.; Catalysis Research Center, Hokkaido University, Sapporo 001-0021, Japan. Control of photocatalytic activity of titania particles by braying and calcination—Effect of electron traps on the photocatalytic activity. Unpublished results. 2014. [Google Scholar]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar]
- Ohtani, B; Prieto-Mahaney, O.O.; Amano, F.; Murakami, N.; Abe, R. What are titania photocatalysts?—An exploratory correlation of photocatalytic activity with structural and physical properties. J. Adv. Oxid. Technol. 2010, 15, 247–261. [Google Scholar]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar]
- Ohtani, B. Titania photocatalysis beyond recombination: A critical review. Catalysts 2013, 3, 942–953. [Google Scholar]
- Nitta, A.; Takase, M.; Ohtani, B. Energy-resolved measurement of electron traps in metal oxide particulate photocatalysts: Reversed double-beam photoacoustic spectroscopy. 2014, in preparation. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Kowalska, E.; Ohtani, B. Influence of Post-Treatment Operations on Structural Properties and Photocatalytic Activity of Octahedral Anatase Titania Particles Prepared by an Ultrasonication-Hydrothermal Reaction. Molecules 2014, 19, 19573-19587. https://doi.org/10.3390/molecules191219573
Wei Z, Kowalska E, Ohtani B. Influence of Post-Treatment Operations on Structural Properties and Photocatalytic Activity of Octahedral Anatase Titania Particles Prepared by an Ultrasonication-Hydrothermal Reaction. Molecules. 2014; 19(12):19573-19587. https://doi.org/10.3390/molecules191219573
Chicago/Turabian StyleWei, Zhishun, Ewa Kowalska, and Bunsho Ohtani. 2014. "Influence of Post-Treatment Operations on Structural Properties and Photocatalytic Activity of Octahedral Anatase Titania Particles Prepared by an Ultrasonication-Hydrothermal Reaction" Molecules 19, no. 12: 19573-19587. https://doi.org/10.3390/molecules191219573






