Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments
Abstract
:1. Introduction
2. Oxidative Environment in a Biological System

3. Sulfur as An Essential Element in Biological System

4. Sulfur Containing Compounds and Their Antioxidant Potentials
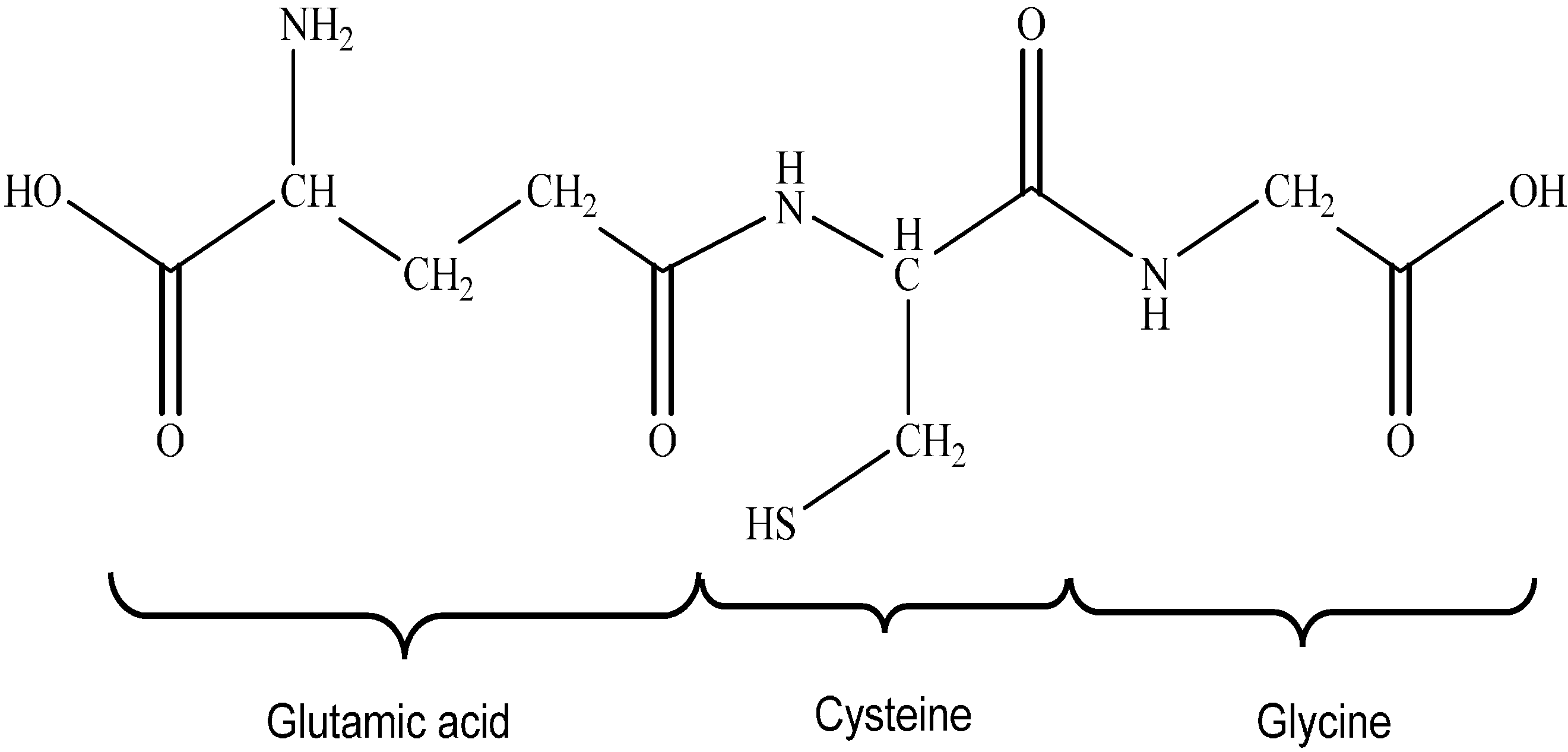
4.1. Glutathione Antioxidant System
Glutathione Synthesis
4.2. Thioredoxin Antioxidant System
4.3. Glutaredoxin Antioxidant System

5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Perez, J.A.M.; Aguilar, T.A.F. Chemistry of Natural Antioxidants and Studies Performed with Different Plants Collected in Mexico, Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Parimala, M.; Shoba, F.G. Phytochemical analysis and in vitro antioxidant activity of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. Asian Pac. J. Trop Biomed. 2013, 3, 887–895. [Google Scholar] [CrossRef]
- Mandal, S.; Yadav, S.; Yadav, S.; Nema, R.K. Antioxidants: A review. J. Chem. Pharm. Res. 2009, 1, 102–104. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Naik, S.R. Antioxidants and their role in biological functions: An overview. Indian Drugs 2003, 40, 501–508. [Google Scholar]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Rad. 2006, 5, 326–334. [Google Scholar]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D. Phenolic antioxidants—radical-scavenging and chain-breaking activity: A comparative study. Eur. J. Lipid Sci. Technol. 2009, 111, 1072–1089. [Google Scholar] [CrossRef]
- Kumar, S. Free Radicals and Antioxidants: Human and Food System. Adv. Appl. Sci. Res. 2011, 2, 129–135. [Google Scholar]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to Use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013. [Google Scholar] [CrossRef]
- Doshi, S.B.; Khullar, K.; Sharma, R.K.; Agarwal, A. Role of reactive nitrogen species in male infertility. Reprod. Biol. Endocrin. 2012, 10, 109. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell. Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.L. Mitochondrial oxygen radical formation during reductive and oxidative stress to intact hepatocytes. Biosci. Rep. 1997, 17, 281–291. [Google Scholar] [CrossRef]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Brand, M.D.; Murhy, M.P. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 2004, 53 (suppl. 1), S110–S118. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar]
- Lia, K.; Yin, M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: Importance of the partition coefficient. J. Agric. Food Chem. 2000, 48, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014, 4, 273–281. [Google Scholar]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, L.J. Protein oxidative modifications: Beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar]
- KR, S.G.; Mathew, B.B.; Sudhamani, C.N.; Naik, H.B. Mechanism of DNA binding and cleavage. J. Biomed. Biotechnol. 2014, 2, 1–9. [Google Scholar]
- Neofytou, E.; Tzortzaki, E.G.; Chatziantoniou, A.; Nikolaos, M.; Siafakas, N.M. DNA damage due to oxidative stress in chronic obstructive pulmonary disease (COPD). Int. J. Mol. Sci. 2012, 13, 16853–16864. [Google Scholar] [CrossRef]
- Ayeleso, A.O.; Brooks, N.L.; Oguntibeju, O.O. Modulation of antioxidant status in streptozotocin-induced diabetic male Wistar rats following intake of red palm oil and/or rooibos. Asian Pac. J. Trop Med. 2014, 536–544. [Google Scholar] [CrossRef]
- Knife, H.H.; Belay, Y.H.; Joseph, J.S.; Mukwevho, E. Evaluation of the influence of thiosemicarbazone-triazole hybrids on genes implicated in lipid oxidation and accumulation as potential anti-obesity agents. Bioorg. Med. Chem. Lett. 2013, 23, 5275–5278. [Google Scholar] [CrossRef] [PubMed]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. 2002, 7, 22–44. [Google Scholar]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J Nutr. 2006, 136 (Suppl. 6), 1636S–1640S. [Google Scholar]
- Manna, P.; Das, J.; Sil, P.C. Role of sulfur containing amino acids as an adjuvant therapy in the prevention of diabetes and its associated complications. Curr. Diabetes Rev. 2013, 9, 237–248. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Szyszka, K.; Szygula, Z. Effect of cysteine derivatives administration in healthy men exposed to intense resistance exercise by evaluation of pro-antioxidant ratio. J. Physiol. Sci. 2007, 57, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Komarnisky, L.A.; Christopherson, R.J.; Basu, T.K. Sulfur: Its clinical and toxicologic aspects. Nutrition 2003, 19, 54–61. [Google Scholar] [CrossRef]
- Varin, L.; Chamberland, H.; Lafontaine, J.G.; Richard, M. The enzyme involved in sulfation of the turgorin, gallic acid 4-O-(β-D-glucopyranosyl-6'-sulfate) is pulvini-localized in Mimosa pudica. Plant. J. 1997, 12, 831–838. [Google Scholar] [CrossRef]
- Varin, L.; Marsolais, F.; Richard, M.; Rouleau, M. Biochemistry and molecular biology of plant sulfotransferases. FASEB J. 1997, 11, 517–525. [Google Scholar]
- Bick, J.A.; Leustek, T. Plant sulfur metabolism—the reduction of sulfate to sulphite. Curr. Opin. Plant. Biol. 1998, 1, 240–244. [Google Scholar]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Lipoic acid protects efficiently only against a specific form of peroxynitrite-induced damage. J. Biol. Chem. 2004, 279, 9693–9697. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [PubMed]
- Sies, H. Glutathione and its role in cellular function. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111–112, 1–14. [Google Scholar]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Grant, C.M.; MacIver, F.H.; Dawes, I.W. Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol. Biol. Cell. 1997, 8, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.L.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanism to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1998. [Google Scholar]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependant enzymes represent a co-ordinately regulated defense against oxidative stress. Free Radical Res. 1999, 31, 273–300. [Google Scholar] [CrossRef]
- Linke, K.; Jakob, U. Not every disulfide lasts forever: Disulfide bond formation as a redox switch. Antioxid. Redox Signal. 2003, 5, 425–434. [Google Scholar]
- Holmgren, A.; Söderberg, B.O.; Eklund, H.; Brändén, C.I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 Å resolution. Proc. Natl. Acad. Sci. USA 1975, 72, 2305–2309. [Google Scholar]
- Štefanková, P.; Kollárová, M.; Barák, I. Thioredoxin—structural and functional complexity. Gen. Physiol. Biophys. 2005, 24, 3–11. [Google Scholar]
- Collet, J.-F.; Messens, J. Structure, function, and mechanism of thioredoxin proteins. Antioxid. Redox. Sign. 2010, 13, 1205–1216. [Google Scholar] [CrossRef]
- Watson, W.H.; Pohl, J.; Montfort, W.R.; Stuchlik, O.; Reed, M.S.; Powis, G.; Jones, D.P. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 2003, 278, 33408–33415. [Google Scholar] [PubMed]
- Nordberg, J.; Arnér, E.S.J. Reactive Oxygen Species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Haendeler, J.; Berk, B.C. Thioredoxin: A key regulator of cardiovascular homeostasis. Circ. Res. 2003, 93, 1029–1033. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin structure and mechanism: Conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 1995, 3, 239–243. [Google Scholar] [CrossRef]
- Chae, H.Z.; Chung, S.J.; Rhee, S.G. Thioredoxin-dependant Peroxide Reductase from Yeast. J. Biol. Chem. 1994, 269, 27670–27678. [Google Scholar] [PubMed]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [PubMed]
- Sagemark, J.; Elgán, T.H.; Bürglin, T.R.; Johansson, C.; Holmgren, A.; Berndt, K.D. Redox properties and evolution of human glutaredoxins. Proteins 2007, 68, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Durgadoss, L.; Valli, R.K.; Joshi, D.C.; Joshi, P.G.; Ravindranath, V. Knockdown of Cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: Implication in neurodegenerative diseases. PLoS One 2008, 3, 1–16. [Google Scholar] [PubMed]
- Mailloux, R.J.; Xuan, J.Y.; McBride, S.; Maharsy, W.; Thorn, S.; Holterman, C.E.; Kennedy, C.R.J.; Rippstein, P.; deKemp, R.; da Silva, J.; et al. Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J. Biol. Chem. 2014, 289, 14812–14828. [Google Scholar]
- Haunhorst, P.; Hanschmann, E.M.; Brautigam, L.; Stehling, O.; Hoffmann, B.; Muhlenhoff, U.; Lill, R.; Berndt, C.; Lillig, C.H. Crucial function of vertabrate glutaredoxin 3 (PICOT) in iron homeostatis and hemoglobin maturation. Mol. Biol. Cell. 2013, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Galloway, J.L.; Barut, B.; Foott, H.; Fraenkel, P.; Axe, J.L.; Weber, G.J.; Dooley, K.; Davidson, A.J.; Schmidt, B.; et al. Deficiency of glutaredoxin 5 reveal Fe-S clusters are required for vertebrate haem synthesis. Nature 2005, 436, 1305–1039. [Google Scholar]
- Grant, C.M. Role of glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 2001, 39, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Antioxidants function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, Glutaredoxins and Peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments. Molecules 2014, 19, 19376-19389. https://doi.org/10.3390/molecules191219376
Mukwevho E, Ferreira Z, Ayeleso A. Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments. Molecules. 2014; 19(12):19376-19389. https://doi.org/10.3390/molecules191219376
Chicago/Turabian StyleMukwevho, Emmanuel, Zané Ferreira, and Ademola Ayeleso. 2014. "Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments" Molecules 19, no. 12: 19376-19389. https://doi.org/10.3390/molecules191219376



