The Antiosteoporotic Activity of Central-Icaritin (CIT) on Bone Metabolism of Ovariectomized Rats
Abstract
:1. Introduction
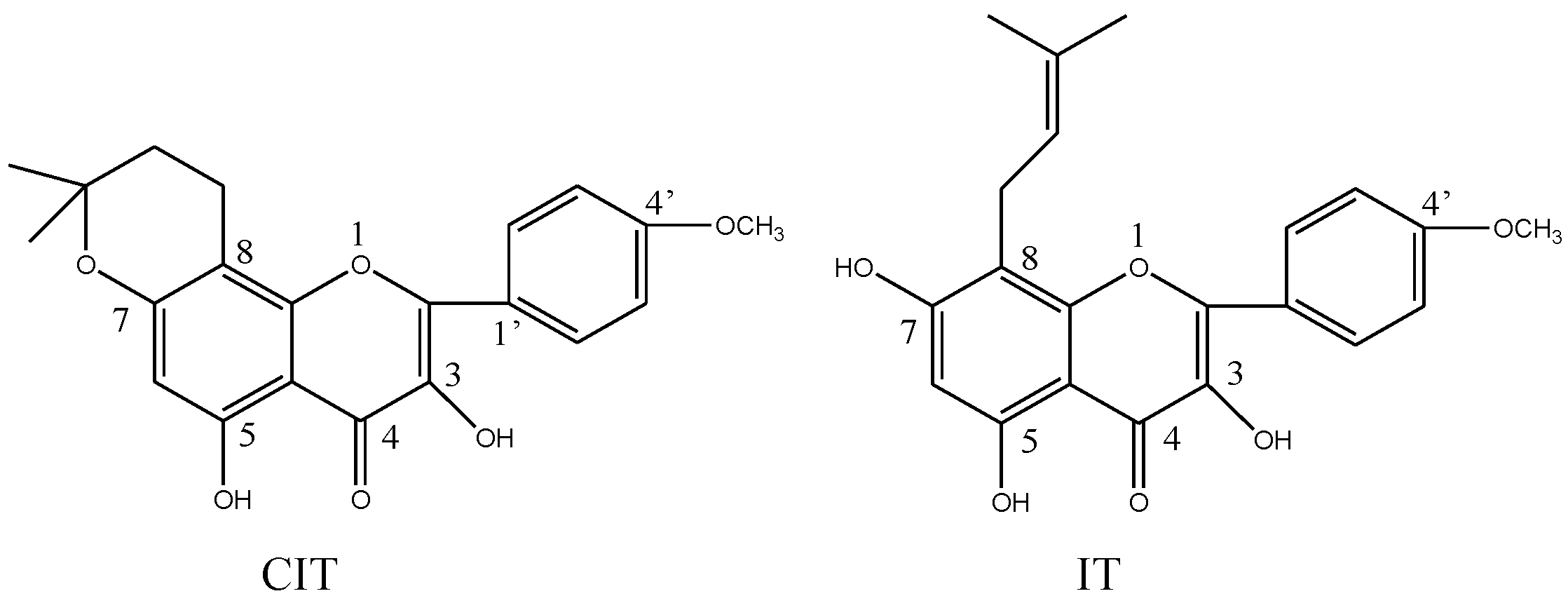
2. Results and Discussion
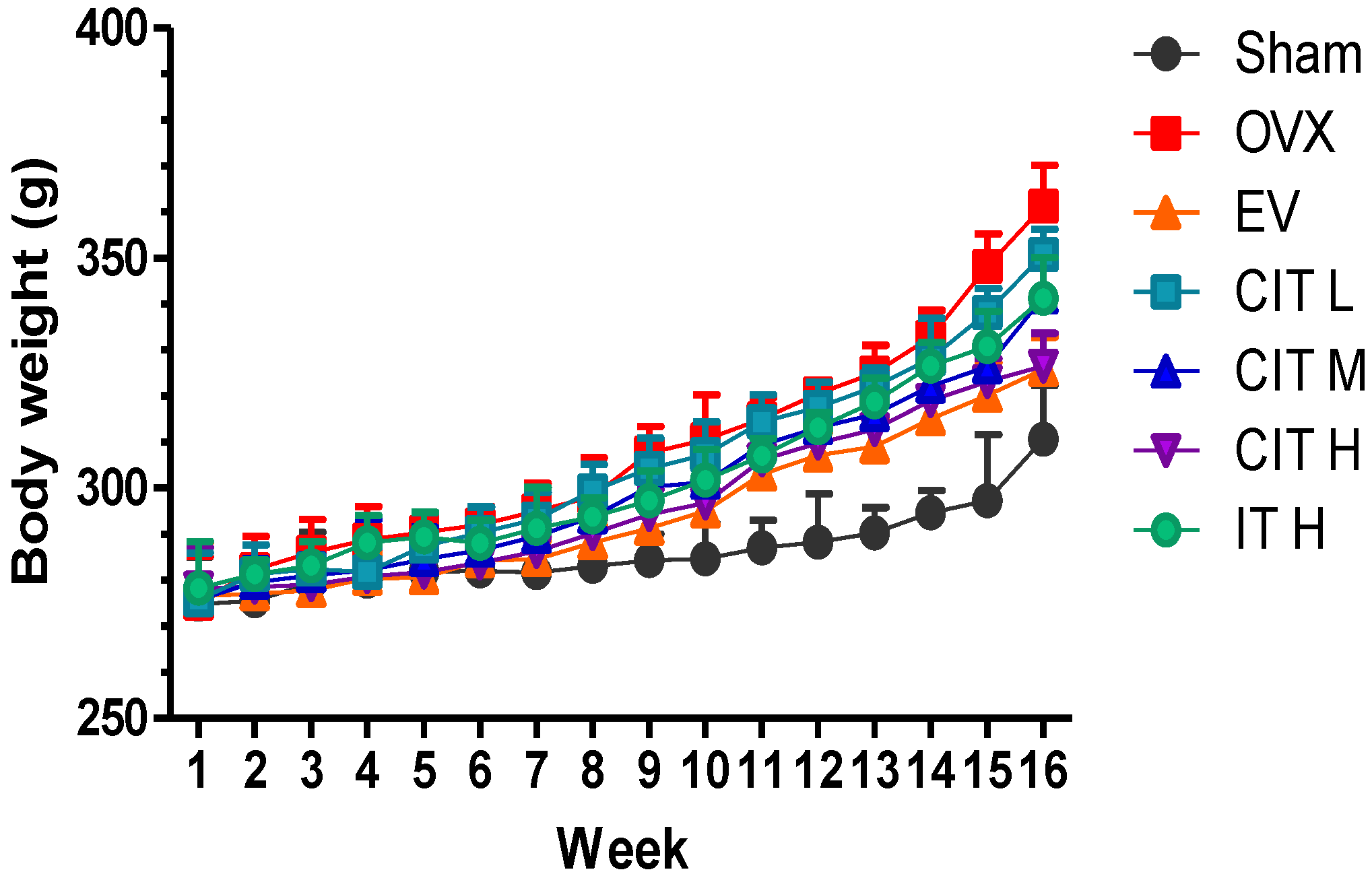
2.1. Body Weight, Femur Weight and Uterus Wet Weight Changes in the OVX Rats
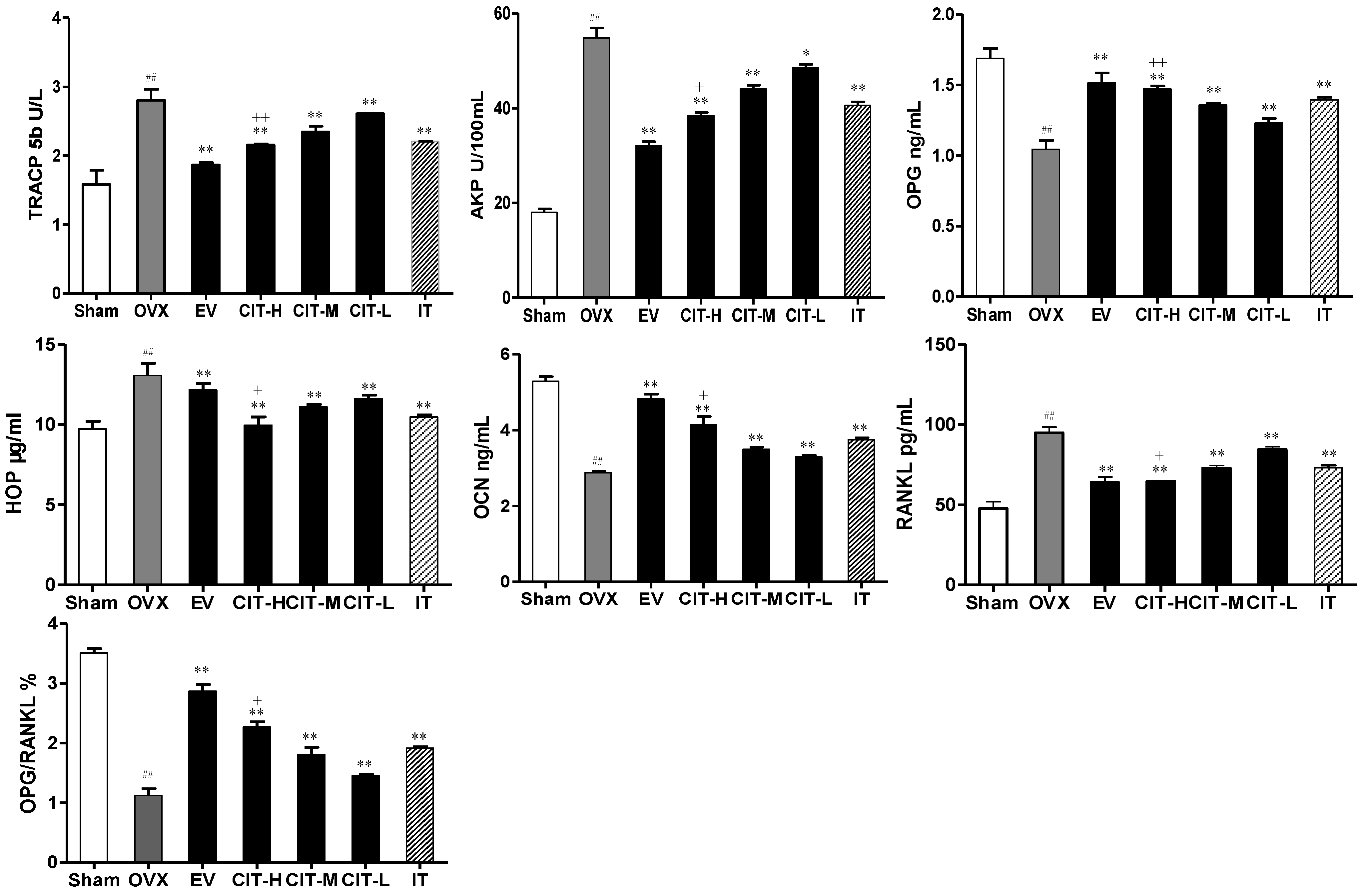
2.2. Serum Biochemical Markers Changes in the OVX Rats
| Weight | Sham | OVX | EV | CIT-L | CIT-M | CIT-H | IT |
|---|---|---|---|---|---|---|---|
| Uterine (g) | 0.783 ± 0.067 | 0.103 ± 0.015 ## | 0.683 ± 0.031 ** | 0.127 ± 0.015 | 0.233 ± 0.051 * | 0.453 ± 0.031 **+ | 0.370 ± 0.02 ** |
| Femur (g) | 0.971 ± 0.040 | 0.741 ± 0.025 ## | 0.894 ± 0.016 ** | 0.777 ± 0.006 | 0.815 ± 0.017 * | 0.845 ± 0.007 **+ | 0.820 ± 0.01 ** |
2.3. Bone Mineral Density (BMD) Changes in the OVX rats
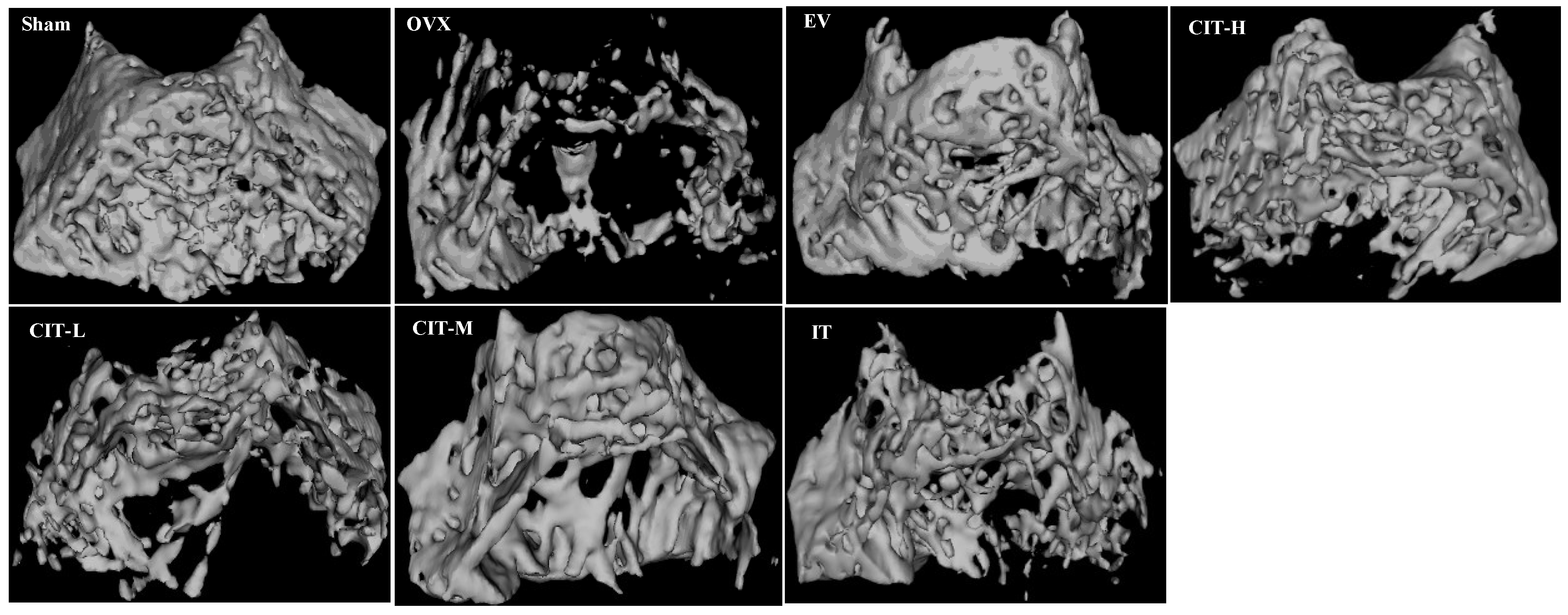
2.4. Micro-CT Analysis in the OVX Rats
| Parameters | Sham | OVX | EV | CIT-L | CIT-M | CIT-H | IT |
|---|---|---|---|---|---|---|---|
| BMD (mg/cm3) | 503.05 ± 16.52 | 357.63 ± 9.26 ## | 431.85 ± 14.50 * | 366.41 ± 9.90 * | 379.42 ± 8.02 * | 392.66 ± 4.58 **+ | 379.12 ± 2.92 * |
| BMC (mg) | 263.84 ± 5.25 | 147.03 ± 6.47 ## | 246.15 ± 10.02 ** | 182.172 ± 4.39 ** | 204.09 ± 7.36 ** | 222.08 ± 3.39 **+ | 203.53 ± 5.71 ** |
| TMC (mg) | 245.16 ± 4.57 | 131.83 ± 3.33 ## | 227.08 ± 3.17 ** | 163.56 ± 6.53 ** | 180.23 ± 8.08 ** | 201.52 ± 3.16 **+ | 193.90 ± 3.01 ** |
| TMD (mg/cm3) | 587.37 ± 2.92 | 488.14 ± 4.62 ## | 579.00 ± 4.77 ** | 509.87 ± 0.10 ** | 515.86 ± 5.49 ** | 554.44 ± 7.37 **++ | 591.33 ± 6.06 ** |
| VOB (mm3) | 448.08 ± 16.78 | 241.25 ±17.07 ## | 415.75 ±12.94 ** | 312.46 ±17.31 ** | 358.19 ±10.16 ** | 382.53 ± 8.98 **+ | 357.97 ± 11.73 ** |
| BS/BV (1/mm) | 2.71 ± 0.09 | 5.44 ± 0.11 ## | 3.41 ± 0.05 ** | 4.55 ± 0.09 ** | 4.06 ± 0.08 ** | 3.77 ± 0.07 **+ | 3.86 ± 0.06 ** |
| BV/TV (%) | 59.95 ± 4.26 | 28.56 ± 1.02 ## | 54.31 ± 4.19 ** | 39.20 ± 1.22 ** | 44.36 ± 1.16 ** | 46.11 ± 0.84 **+ | 44.42 ± 0.44 ** |
| Tb.Th (mm) | 0.71 ± 0.02 | 0.33 ± 0.02 ## | 0.64 ± 0.03 ** | 0.36 ± 0.01 * | 0.44 ± 0.01 ** | 0.47 ± 0.02 **+ | 0.43 ± 0.01 ** |
| Tp.Sp (mm) | 0.24 ± 0.01 | 0.69 ± 0.03 ## | 0.32 ± 0.01 ** | 0.61 ± 0.01 * | 0.45 ± 0.01 ** | 0.41 ± 0.02 **+ | 0.45 ± 0.02 ** |
| Tb.N (1/mm) | 6.11 ± 0.91 | 1.68 ± 0.06 ## | 4.13 ± 0.32 ** | 2.01 ± 0.13 * | 2.74 ± 0.12 ** | 3.65 ± 0.17 **+ | 3.20 ± 0.12 ** |
| Calib.Tb.Th (mm) | 0.76 ± 0.04 | 0.44 ± 0.04 ## | 0.67 ± 0.01 ** | 0.50 ± 0.01 | 0.57 ± 0.01 * | 0.61 ± 0.02 *+ | 0.58 ± 0.01 ** |
| Calib.Tb.Sp (mm) | 0.22 ± 0.01 | 0.58 ± 0.01 ## | 0.30 ±0.01** | 0.53 ± 0.01 ** | 0.42 ± 0.01 ** | 0.35 ± 0.02 **++ | 0.41 ± 0.01 ** |
| Parameters | Sham | OVX | EV | CIT-L | CIT-M | CIT-H | IT |
|---|---|---|---|---|---|---|---|
| Ultimate load (N) | 94.66 ± 3.99 | 63.11 ± 2.69 ## | 88.76 ± 1.72 ** | 68.38 ± 1.73 * | 76.10 ± 1.57 * | 81.47 ± 1.94 *+ | 75.56 ± 1.26 ** |
| Stiffness (N/mm) | 247.27 ± 17.20 | 146.29 ± 12.33 ## | 224.02 ± 6.38 ** | 164.37 ± 10.87 | 177.48 ± 8.53 * | 196.25 ± 7.03 **+ | 180.69 ± 5.53 ** |
| Energy to failure (mJ) | 23.37 ± 2.02 | 11.51 ± 1.16 ## | 21.07 ± 0.62 ** | 13.99 ± 1.00 * | 16.94 ± 0.98 ** | 18.92 ± 0.47 **+ | 16.84 ± 1.10 ** |
2.5. Three-Point Bending Test in the OVX Rats
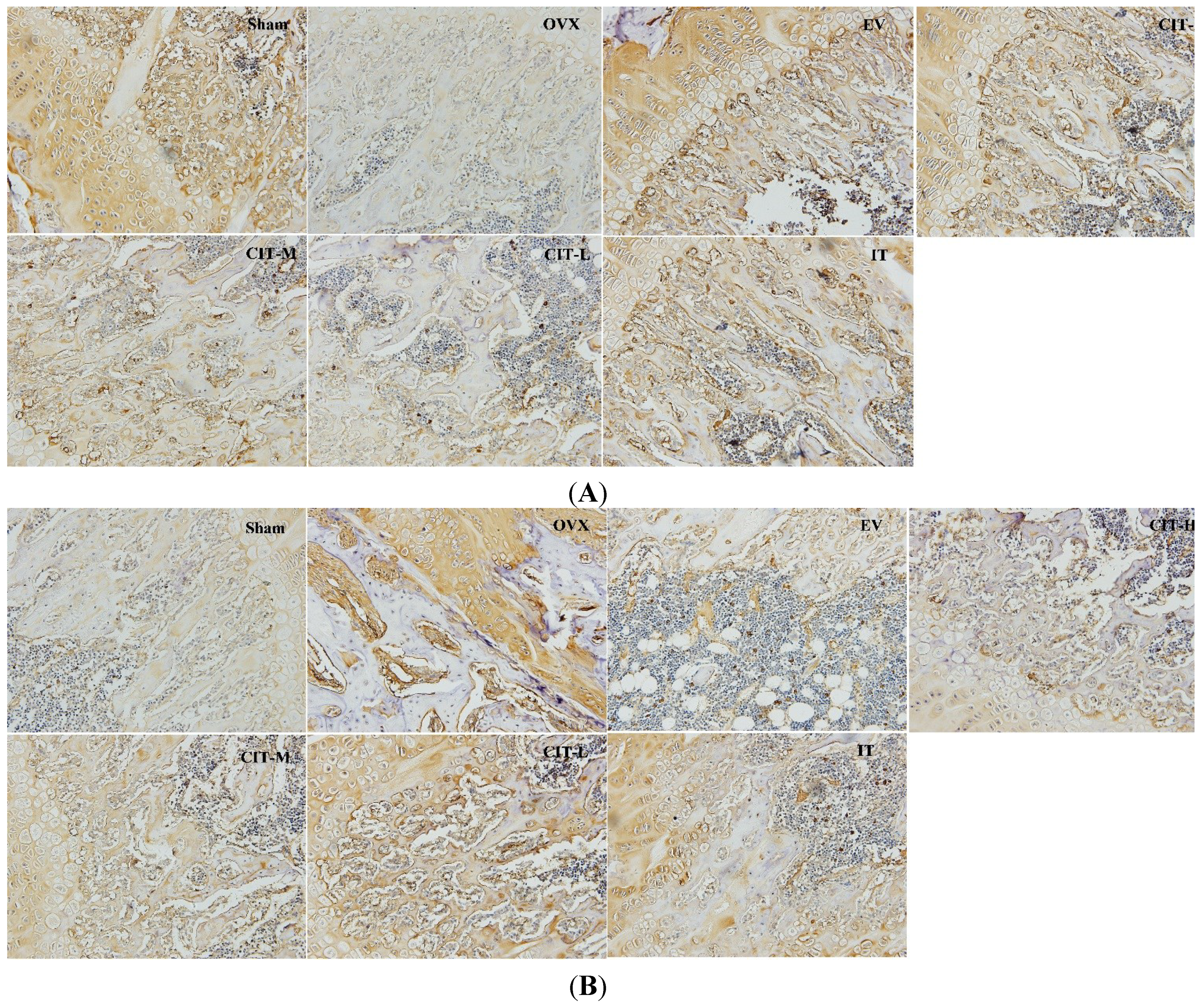
2.6. Immunohistochemical Analysis in the OVX Rats
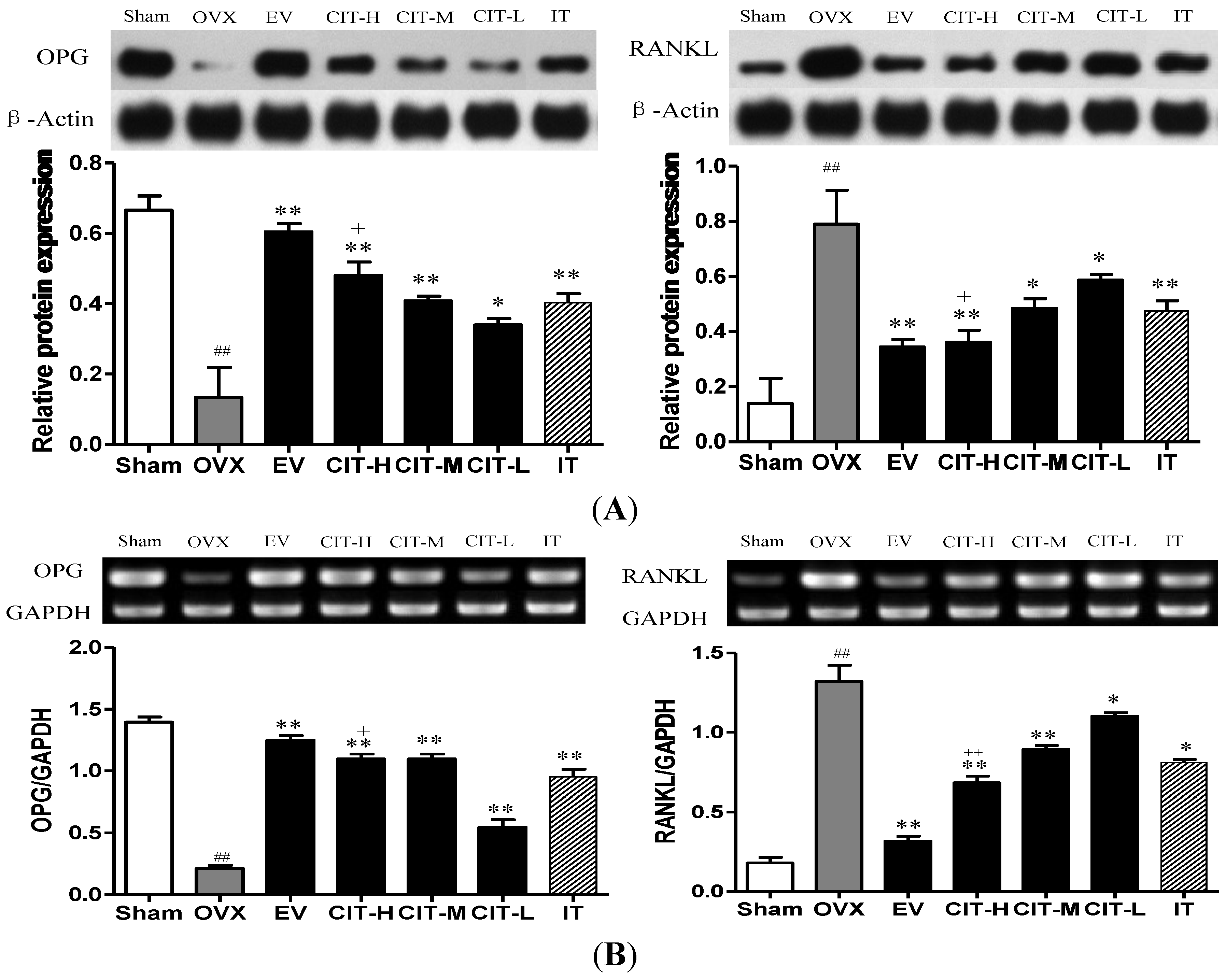
2.7. Expressions of OPG and RANKL in the OVX Rats
2.8. Discussion
3. Experimental Section
3.1. Animals and Reagents
3.2. Serum Parameters
3.3. Measurement of Bone Mineral Density (BMD)
3.4. Micro-CT Analysis
3.5. Biomechanical Evaluation
3.6. Immunohistochemical Staining
3.7. Real-Time PCR
3.8. Western Blot Assays
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, D.; Peng, S.; Yang, S.H.; Shao, Z.W.; Yang, C.; Feng, Y.; Wu, W.; Zhen, W.X. The beneficial effect of Icariin on bone is diminished in osteoprotegerin deficient mice. Bone 2012, 51, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Su, I.H.; Chen, Y.C.; Hwang, W.T.; Liu, Z.; Su, T.P.; Chen, T.J.; Barnhart, K.T.; Yang, Y.X. Risks and benefits of menopausal hormone therapy in postmenopausal Chinese women. Menopause 2012, 19, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P. Menopausal hormone therapy has risks and benefits during the intervention and poststopping phase. Evid. Based Med. 2014, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.; Tamboli, C.; Tamboli, S.; Acosta, S.; De, L.P.I.; Sanberg, P.R.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Estrogen replacement therary for stroke. Cell Med. 2014, 6, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Neville, W.H.L.; Coleman, R.E. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur. J. Cancer 2010, 46, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.G.; Cheng, L.; Li, K.H.; Liu, W.H.; Xu, M.; Jiang, W.; Wei, L.C.; Zhang, F.J.; Xiao, W.F.; Xiong, Y.L.; et al. Effect of epimedium pubescen flavonoid on bone mineral status and bone turnover in male rats chronically exposed to cigarette smoke. BMC Musculoskelet. Disord. 2012, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.M.; Zhang, P.; Huang, H.; Shen, Y.G.; Yang, Q.H.; Cui, W.L.; He, Y.S.; Wei, S.; Ye, Z.; Liu, F.; et al. Five-year follow-up study of a kidney-tonifying herbal Fufang for prevention of postmenopausal osteoporosis and fragility fractures. J. Bone Miner. Metab. 2012, 30, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, G.; He, Y.; Wang, X.; Leung, P.; Leung, K.; Qin, L. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone 2009, 45, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, Y.H.; Kwon, H.; Lee, S.B.; Kim, D.H.; Sung, C.K.; Park, Y.I.; Dong, M.S. Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components: In vitro and in vivo studies. Food Chem. Toxicol. 2012, 50, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- French, D.L.; Muir, J.M.; Webber, C.E. The ovariectomized, mature rat model of postmenopausal osteoporosis: An assessment of the bone sparing effects of curcumin. Phytomedicine 2008, 15, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Nian, H.; Ma, M.H.; Nian, S.S.; Xu, L.L. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine 2009, 16, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, B.; Li, Q.; Peng, J.; Yang, Z. miR-214 targets ATF4 to inhibit bone formation. Nat. Med. 2013, 19, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Laib, A.; Kumer, J.L.; Majumdar, S.; Lane, N.E. The temporal changes of trabecular architecture in ovariectomized rats assessed by Micro CT. Osteoporos. Int. 2001, 12, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Meng, J.; Jia, M.; Bi, L.; Zhou, Y.; Wang, Y.; Hu, J.; He, G.; Luo, X. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J. Bone Miner. Res. 2013, 28, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Z.; Chen, H.K.; Simmons, H.A.; Qi, H.; Crawford, D.T.; Pirie, C.M.; Chidsey-Frink, K.L.; Ma, Y.F.; Jee, W.S.; Thompson, D.D. Comparative effects of droloxifene, tamoxifen, and estrogen on bone, serum cholesterol, and uterine histology in the ovariectomized rat model. Bone 1997, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Lou, Y.J. Estrogenic effects of two derivatives of icariin on human breast cancer MCF-7 cells. Phytomedicine 2005, 12, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Zhou, J.; Gao, X.; Qu, D.; Liu, C. Study on the mechanism of intestinal absorption of epimedins a, B and C in the Caco-2 cell model. Molecules 2014, 19, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Sun, E.; Zhenhai, Z.; Qian, Q.; Tan, X.; Xu, F.; Jia, X. Metabolite profiles of epimedin B in rats by ultraperformance liquid chromatography quadrupole-time-of-flight mass spectrometry. J. Agric. Food Chem. 2013, 61, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Li, S.L.; Sun, E.; Zhang, K.R.; Tan, X.B.; Wei, Y.J.; Fan, H.W.; Cui, L.; Jia, X.B. Metabolite profiles of icariin in rat plasma by ultra-fast liquid chromatography coupled to triple-quadrupole/time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.E.; Nelson, C.; Waring, M.A. Metabolites of dietary (soya) isoflavones in humans urine. Clin. Chim. Acta. 1993, 223, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, G.N.; Li, Y.; Zhang, L.; You, C.; Zheng, Y. Oral absorption and excretion of icaritin, an aglycone and also active metabolite of prenylflavonoids from the Chinese medicine Herba Epimedii in rats. Phytomedicine 2012, 19, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Feng, L.; Sun, E.; Li, H.; Cui, L.; Xiaobin, Jia. Metabolic profiling of isomeric aglycones central-icaritin (c-IT) and icaritin (IT) in osteoporotic rats by UPLC-QTOF-MS. Drug Test. Anal. 2014. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, G.; Zhang, B.T.; Guo, B.; He, Y.; Bakker, A.J.; Pan, X.; Zhen, W.; Hung, L.; Qin, L.; et al. The beneficial effect of Icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone 2013, 55, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Tuukkanen, J.; Zhang, H. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone 1994, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds CIT and IT are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Li, J.; Jia, X. The Antiosteoporotic Activity of Central-Icaritin (CIT) on Bone Metabolism of Ovariectomized Rats. Molecules 2014, 19, 18690-18704. https://doi.org/10.3390/molecules191118690
Jiang J, Li J, Jia X. The Antiosteoporotic Activity of Central-Icaritin (CIT) on Bone Metabolism of Ovariectomized Rats. Molecules. 2014; 19(11):18690-18704. https://doi.org/10.3390/molecules191118690
Chicago/Turabian StyleJiang, Jun, Jie Li, and Xiaobin Jia. 2014. "The Antiosteoporotic Activity of Central-Icaritin (CIT) on Bone Metabolism of Ovariectomized Rats" Molecules 19, no. 11: 18690-18704. https://doi.org/10.3390/molecules191118690




