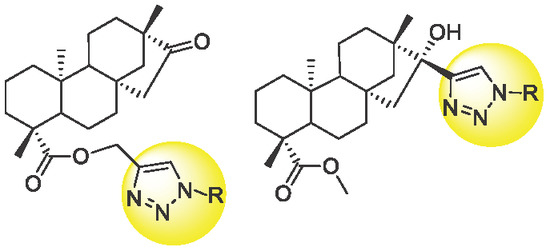
Design and Synthesis of Isosteviol Triazole Conjugates for Cancer Therapy
Abstract
:1. Introduction
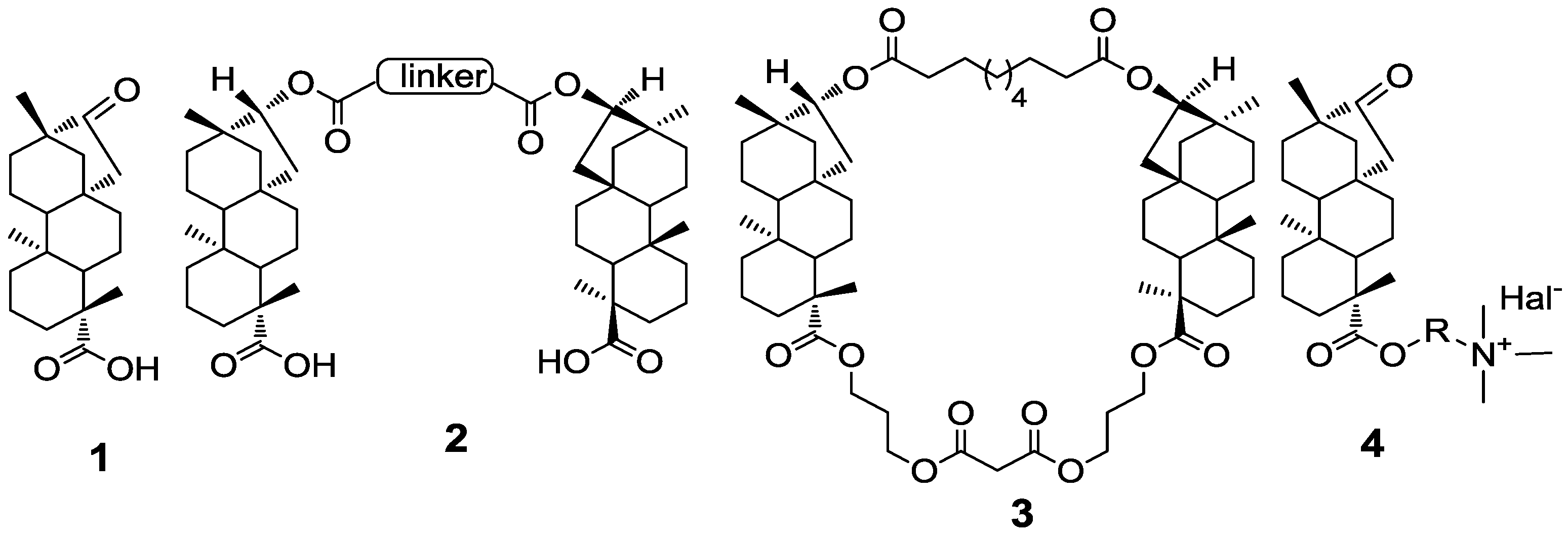
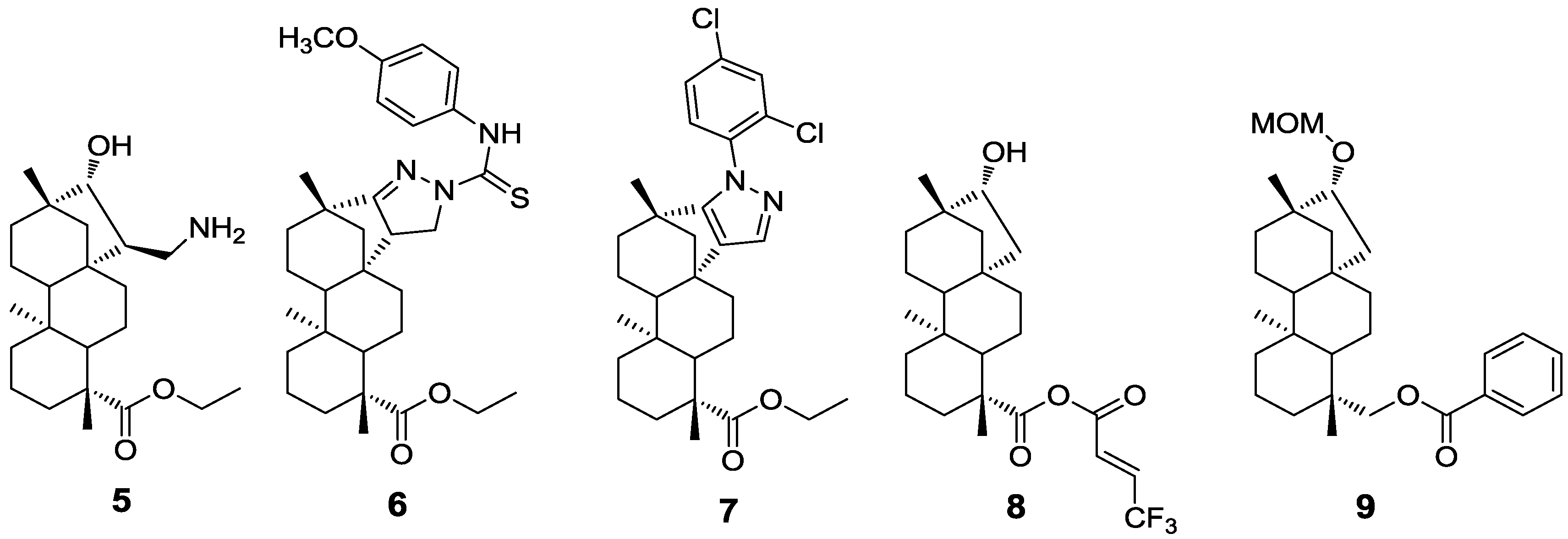
2. Results and Discussion
2.1. Design and Synthesis of Alkyne Derivatives of Isosteviol.
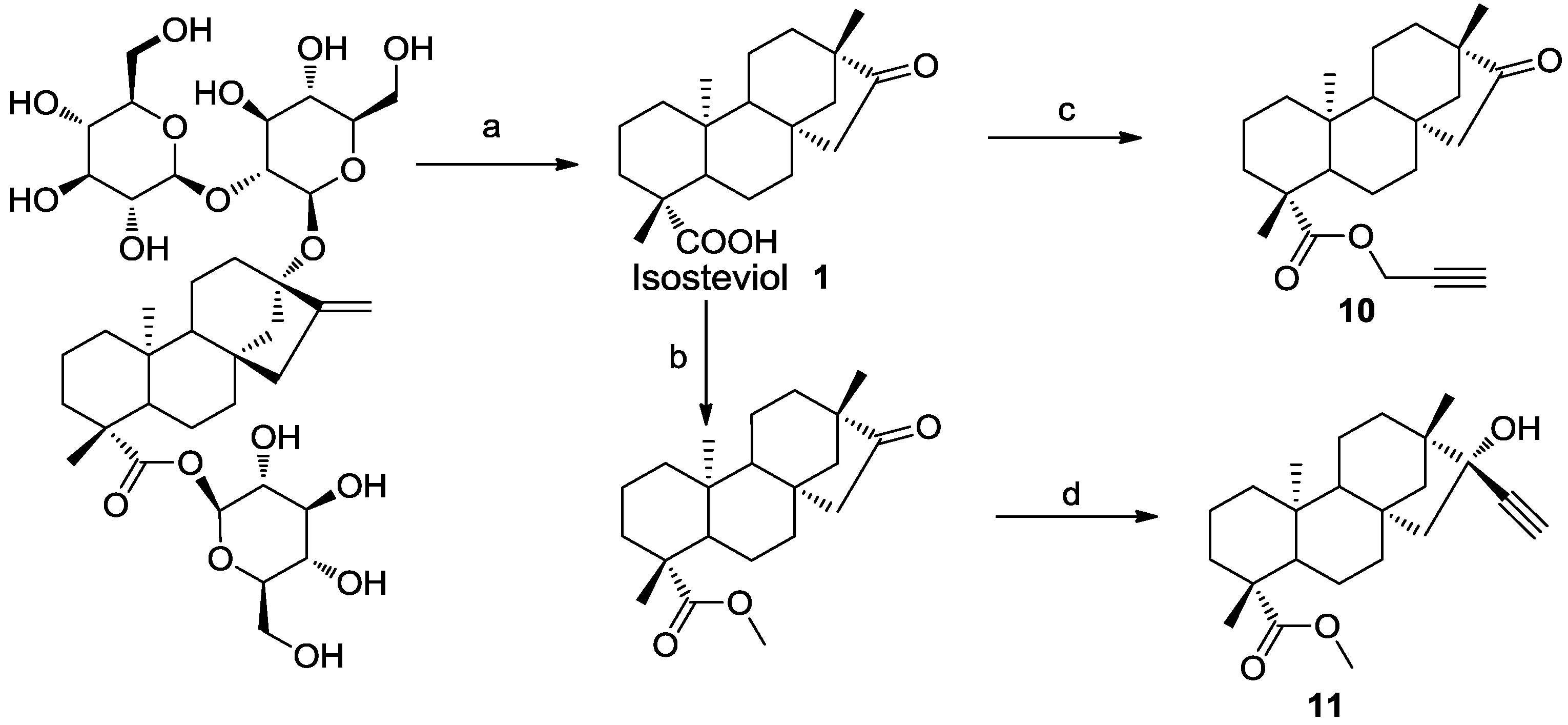
2.2. Design and Synthesis of the Isosteviol Conjugates

2.3. Evaluation of Cytotoxic Activity
| Compound | IC50 (µM) | |||||
|---|---|---|---|---|---|---|
| A549 (Lung) | ASPC-1 (Pancreas) | MDA231 (Breast) | PC-3 (Prostate) | HCT116 (Colon) | HeLa (Cervical) | |
| 12a | 9.95 ± 0.24 | 4.79 ± 0.18 | 13.76 ± 0.63 | 18.23 ± 0.95 | 6.60 ± 0.38 | 20.18 ± 1.03 |
| 12b | 98.42 ± 5.63 | >100 | >100 | >100 | 13.66 ± 0.41 | 5.83 ± 0.33 |
| 12c | 63.71 ± 3.84 | >100 | >100 | >100 | >100 | 51.14 ± 3.65 |
| 12d | >100 | >100 | >100 | >100 | >100 | >100 |
| 13a | 43.52 ± 1.52 | >100 | 69.2 ± 5.23 | >100 | 45.95 ± 2.33 | 29.62 ± 1.52 |
| 13b | 31.7 ± 1.84 | >100 | 38.12 ± 1.82 | 73.65 ± 5.61 | 37.71 ± 1.03 | 30.72 ± 0.62 |
| 13c | 56.4 ± 1.98 | 54.68 ± 3.28 | 50.13 ± 3.08 | 43.84 ± 1.33 | 48.9 ± 1.93 | 40.57 ± 2.81 |
| 13d | >100 | >100 | >100 | >100 | >100 | >100 |

| Compound | IC50 (µM) | Compound | IC50 (µM) | ||
|---|---|---|---|---|---|
| MOLT-4 (Leukemia) | HL-60 (Leukemia) | MOLT-4 (Leukemia) | HL-60 (Leukemia) | ||
| 12a | 5.02 ± 0.15 | 28.8 ± 0.63 | 13a | 12.8 ± 0.54 | 42.38 ± 2.89 |
| 12b | 7.27 ± 0.32 | 35.68 ± 1.08 | 13b | 21.04 ± 1.03 | 63.62 ± 3.02 |
| 12c | 35.49 ± 0.77 | 46.77 ± 1.47 | 13c | 31.26 ± 1.28 | 32.89 ± 1.87 |
| 12d | >100 | >100 | 13d | >100 | >100 |
3. Experimental Section
3.1. General Procedures
3.2. Synthesis
3.2.1. Preparation of Alkyne Isosteviol (10)
3.2.2. General Procedures for the Preparation of Conjugates by Copper(I)-catalyzed Azide-alkyne Cycloaddition Reaction
3.3. Biological Materials
3.4. Cell Culture and MTT Assay
3.5. Cell Titer Glo Assay
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Kao, P.F.; Hsieh, M.H.; Chen, Y.J.; Chan, P. The antihypertensive effects of stevioside derivative isosteviol in spontaneously hypertensive rats. Acta Cardiol. Sin. 2001, 17, 133–140. [Google Scholar]
- Wong, K.L.; Yang, H.Y.; Chan, P.; Cheng, T.H.; Liu, J.C.; Hsu, F.L.; Liu, I.M.; Cheng, Y.W.; Cheng, J.T. Isosteviol as a potassium channel opener to lower intracellular calcium concentrations in cultured aortic smooth muscle cells. Planta Med. 2004, 70, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Z.; Wang, J.; Milne, R.W.; Xu, D.; Davey, A.K.; Evans, A.M. Isosteviol reduces plasma glucose levels in the intravenous glucose tolerance test in zucker diabetic fatty rats. Diabetes Obes. Metab. 2007, 9, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Lin, J.W.; Liu, J.C.; Yang, H.Y.; Kao, P.F.; Chen, C.H.; Loh, S.H.; Chiu, W.T.; Cheng, T.H.; Lin, J.G.; et al. Antiproliferative effect of isosteviol on angiotensin-ii-treated rat aortic smooth muscle cells. Pharmacology 2006, 76, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Du, W.; Zhao, L.; Davey, A.K.; Wang, J. The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Med. 2008, 74, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Moons, N.; de Borggraeve, W.; Dehaen, W. Isosteviol as a starting material in organic synthesis. Curr. Org. Chem. 2011, 15, 2731–2741. [Google Scholar] [CrossRef]
- Lohoelter, C.; Weckbecker, M.; Waldvogel, S.R. (−)-isosteviol as a versatile ex-chiral-pool building block for organic chemistry. Eur. J. Org. Chem. 2013, 2013, 5539–5554. [Google Scholar] [CrossRef]
- Kataev, V.E.; Khaybullin, R.N.; Sharipova, R.R.; Strobykina, I.Y. Ent-kaurane diterpenoids and glycosides: Isolation, properties, and chemical transformations. Rev. J. Chem. 2011, 1, 93–160. [Google Scholar] [CrossRef]
- Hutt, O.E.; Doan, T.L.; Georg, G.I. Synthesis of skeletally diverse and stereochemically complex library templates derived from isosteviol and steviol. Org. Lett. 2013, 15, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Roberts, F.G.; Wilderman, P.R.; Zhou, K.; Peters, R.J.; Coates, R.M. 16-aza-ent-beyerane and 16-aza-ent-trachylobane: Potent mechanism-based inhibitors of recombinant ent-kaurene synthase from arabidopsis thaliana. J. Am. Chem. Soc. 2007, 129, 12453–12460. [Google Scholar] [CrossRef] [PubMed]
- Kataev, V.E.; Militsina, O.I.; Strobykina, I.Y.; Kovylyaeva, G.I.; Musin, R.Z.; Fedorova, O.V.; Rusinov, G.L.; Zeuva, M.N.; Mordovskoi, G.G.; Tolstikov, A.G. Synthesis and anti-tuberculous activity of diesters based on isosteviol and dicarboxylic acids. Pharm. Chem. J. 2006, 40, 473–475. [Google Scholar] [CrossRef]
- Khaybullin, R.N.; Strobykina, I.Y.; Dobrynin, A.B.; Gubaydullin, A.T.; Chestnova, R.V.; Babaev, V.M.; Kataev, V.E. Synthesis and antituberculosis activity of novel unfolded and macrocyclic derivatives of ent-kaurane steviol. Bioorg. Med. Chem. Lett. 2012, 22, 6909–6913. [Google Scholar] [CrossRef] [PubMed]
- Korochkina, M.G.; Nikitashina, A.D.; Khaybullin, R.N.; Petrov, K.A.; Strobykina, I.Y.; Zobov, V.V.; Kataev, V.E. Unfolded and macrocyclic ammonium derivatives of diterpenoids steviol and isosteviol having choline moieties. Synthesis and inhibitory activities toward acetylcholine- and butyrylcholinesterases. Med. Chem. Commun. 2012, 3, 1449–1454. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, L.H.; Liu, H.; Wang, J.W.; Wang, R.X.; Zhang, Y.X.; Tao, J.C. D-ring modified novel isosteviol derivatives: Design, synthesis and cytotoxic activity evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 5827–5832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Wu, Y.; Liu, C.J.; Wei, C.Y.; Tao, J.C.; Liu, H.M. Design and stereoselective synthesis of novel isosteviol-fused pyrazolines and pyrazoles as potential anticancer agents. Eur. J. Med. Chem. 2013, 65, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Sawada, S.; Kikuchi, T.; Kushi, Y.; Fukatsu, M.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of steviol and isosteviol derivatives against human cancer cell lines. Chem. Biodivers. 2013, 10, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; Laha, R.; Bergmeier, S.C. Synthesis and cytotoxic activity of mom-ether analogs of isosteviol. Bioorg. Med. Chem. Lett. 2014, 24, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.E.; Lecaille, F.; Lalmanach, G.; Aucagne, V.; Delmas, A.F. Synthesis of a biologically active triazole-containing analogue of cystatin a through successive peptidomimetic alkyne-azide ligations. Angew. Chem. Int. Ed. Engl. 2012, 51, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Wang, Y.; Liu, L.; Weng, X.; Li, G.; Zhang, X.; Zhou, X. Novel anthraquinone derivatives: Synthesis via click chemistry approach and their induction of apoptosis in bgc gastric cancer cells via reactive oxygen species (ros)-dependent mitochondrial pathway. Bioorg. Med. Chem. Lett. 2008, 18, 6505–6508. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Tintori, C.; Furlan, A.; Borrelli, S.; Christodoulou, M.S.; Dono, R.; Maina, F.; Botta, M.; Amat, M.; Bosch, J.; et al. “Click” synthesis of a triazole-based inhibitor of met functions in cancer cells. Bioorg. Med. Chem. Lett. 2012, 22, 4693–4696. [Google Scholar] [CrossRef] [PubMed]
- Arioli, F.; Borrelli, S.; Colombo, F.; Falchi, F.; Filippi, I.; Crespan, E.; Naldini, A.; Scalia, G.; Silvani, A.; Maga, G.; et al. N-[2-methyl-5-(triazol-1-yl)phenyl]pyrimidin-2-amine as a scaffold for the synthesis of inhibitors of bcr-abl. ChemMedChem 2011, 6, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, Z.; Zhong, H.; Geng, D.; Zhang, M.; Zhang, T.; Li, Y.; Li, K. Convenient synthesis of novel geiparvarin analogs with potential anti-cancer activity via click chemistry. Eur. J. Med. Chem. 2012, 53, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Verdugo, V.; Theoduloz, C.; Schmeda-Hirschmann, G. Synthesis and antiproliferative activity of some novel triazole derivatives from dehydroabietic acid. Molecules 2014, 19, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Dar, B.A.; Majeed, R.; Hamid, A.; Bhat, B.A. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2013, 66, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Avent, A.G.; Hanson, J.R.; Deoliveira, B.H. Hydrolysis of the diterpenoid glycoside, stevioside. Phytochemistry 1990, 29, 2712–2715. [Google Scholar] [CrossRef]
- Moons, N.; Goyens, D.; Jacobs, J.; van Meervelt, L.; De Borggraeve, W.M.; Dehaen, W. Fused derivatives of (iso)steviol via pericyclic reactions. Tetrahedron Lett. 2012, 53, 6806–6809. [Google Scholar] [CrossRef]
- Shao, C.; Wang, X.; Zhang, Q.; Luo, S.; Zhao, J.; Hu, Y. Acid-base jointly promoted copper(I)-catalyzed azide-alkyne cycloaddition. J. Org. Chem. 2011, 76, 6832–6836. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Verduyn, L.S.; Mirfeizi, L.; Dierckx, R.A.; Elsinga, P.H.; Feringa, B.L. Phosphoramidite accelerated copper(I)-catalyzed [3+2] cycloadditions of azides and alkynes. Chem. Commun. 2009, 2139–2141. [Google Scholar]
- Rijkers, D.T.S.; van Vugt, H.H.R.; Jacobs, H.J.F.; Liskamp, R.M.J. A convenient synthesis of azido peptides by post-assembly diazo transfer on the solid phase applicable to large peptides. Tetrahedron Lett. 2002, 43, 3657–3660. [Google Scholar] [CrossRef]
- Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. Engl. 2005, 44, 5188–5240. [Google Scholar] [CrossRef] [PubMed]
- Scriven, E.F.V.; Turnbull, K. Azides—Their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Bräse, S.; Banert, K. Part 1. Lab-scale synthesis of azido compounds: Safety measures and analysis. In Organic Azides: Syntheses and Applications, 1st ed.; John Wiley & Sons: West Sussex, UK, 2009; Volume 1, pp. 3–27. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors with official material transfer agreement form.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaybullin, R.N.; Zhang, M.; Fu, J.; Liang, X.; Li, T.; Katritzky, A.R.; Okunieff, P.; Qi, X. Design and Synthesis of Isosteviol Triazole Conjugates for Cancer Therapy. Molecules 2014, 19, 18676-18689. https://doi.org/10.3390/molecules191118676
Khaybullin RN, Zhang M, Fu J, Liang X, Li T, Katritzky AR, Okunieff P, Qi X. Design and Synthesis of Isosteviol Triazole Conjugates for Cancer Therapy. Molecules. 2014; 19(11):18676-18689. https://doi.org/10.3390/molecules191118676
Chicago/Turabian StyleKhaybullin, Ravil N., Mei Zhang, Junjie Fu, Xiao Liang, Tammy Li, Alan R. Katritzky, Paul Okunieff, and Xin Qi. 2014. "Design and Synthesis of Isosteviol Triazole Conjugates for Cancer Therapy" Molecules 19, no. 11: 18676-18689. https://doi.org/10.3390/molecules191118676