Rational Design and Synthesis of Altered Peptide Ligands based on Human Myelin Oligodendrocyte Glycoprotein 35–55 Epitope: Inhibition of Chronic Experimental Autoimmune Encephalomyelitis in Mice
Abstract
:1. Introduction
2. Results and Discussion


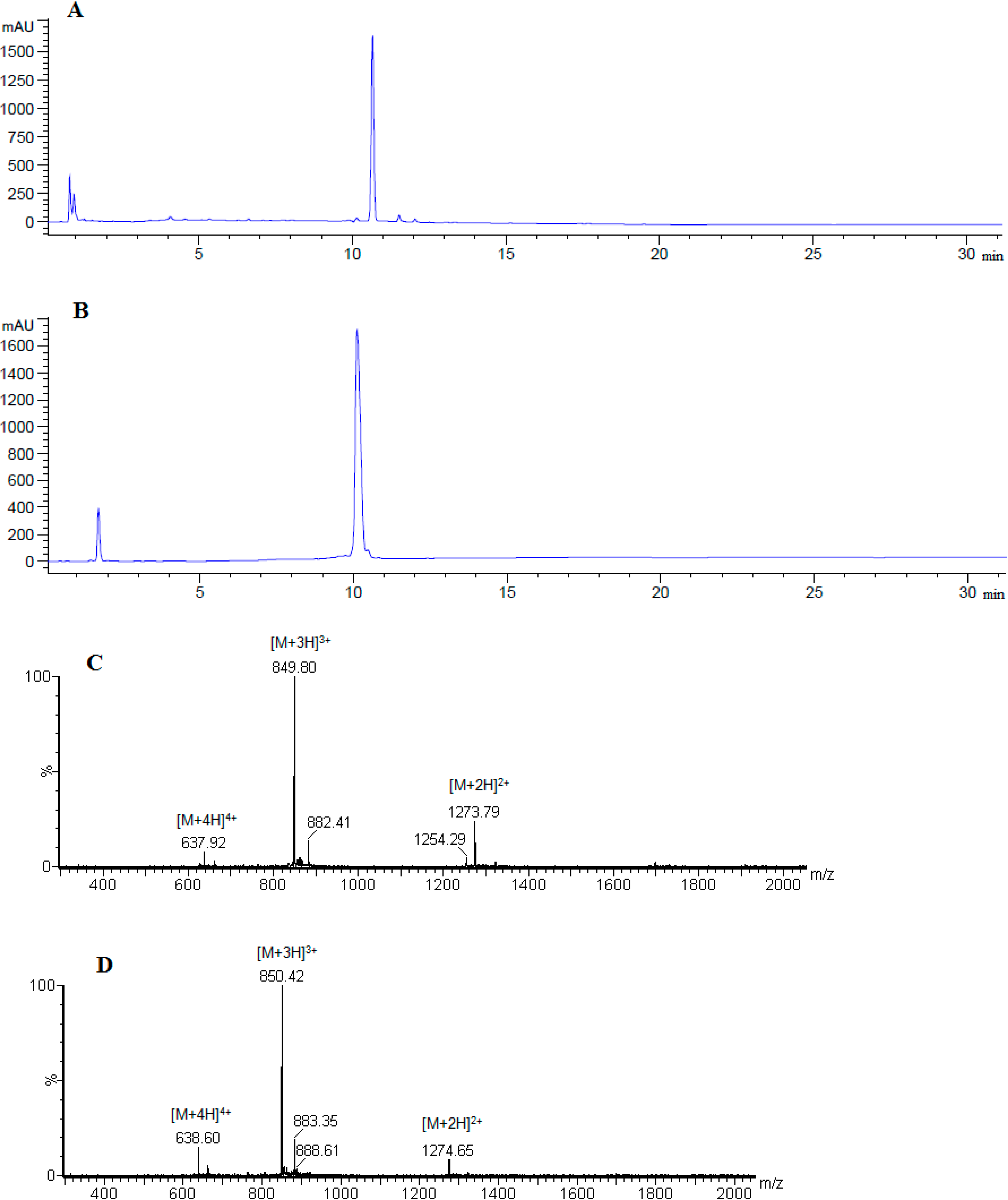
| No. | Peptide | tR (min) | HPLC Purity % # | HPLC Purity % ≠ | Mcalc. (Da) |
|---|---|---|---|---|---|
| 1 | [Ala41]MOG35–55 | 10.23 | 75.3 | 98.2 | 2505.27 |
| 2 | [Ala41,46]MOG35–55 | 10.89 | 74.3 | 98.9 | 2420.21 |
| 3 | [TyrOMe40]MOG35–55 | 09.16 | 58.2 | 98.7 | 2603.35 |
| 4 | cyclo(46–55)MOG35–55 | 10.57 | 29.1 | 97.7 | 2545.97 |
| 5 | cyclo(41–55)MOG35–55 | 09.96 | 31.8 | 97.9 | 2545.97 |
2.1. Chemistry
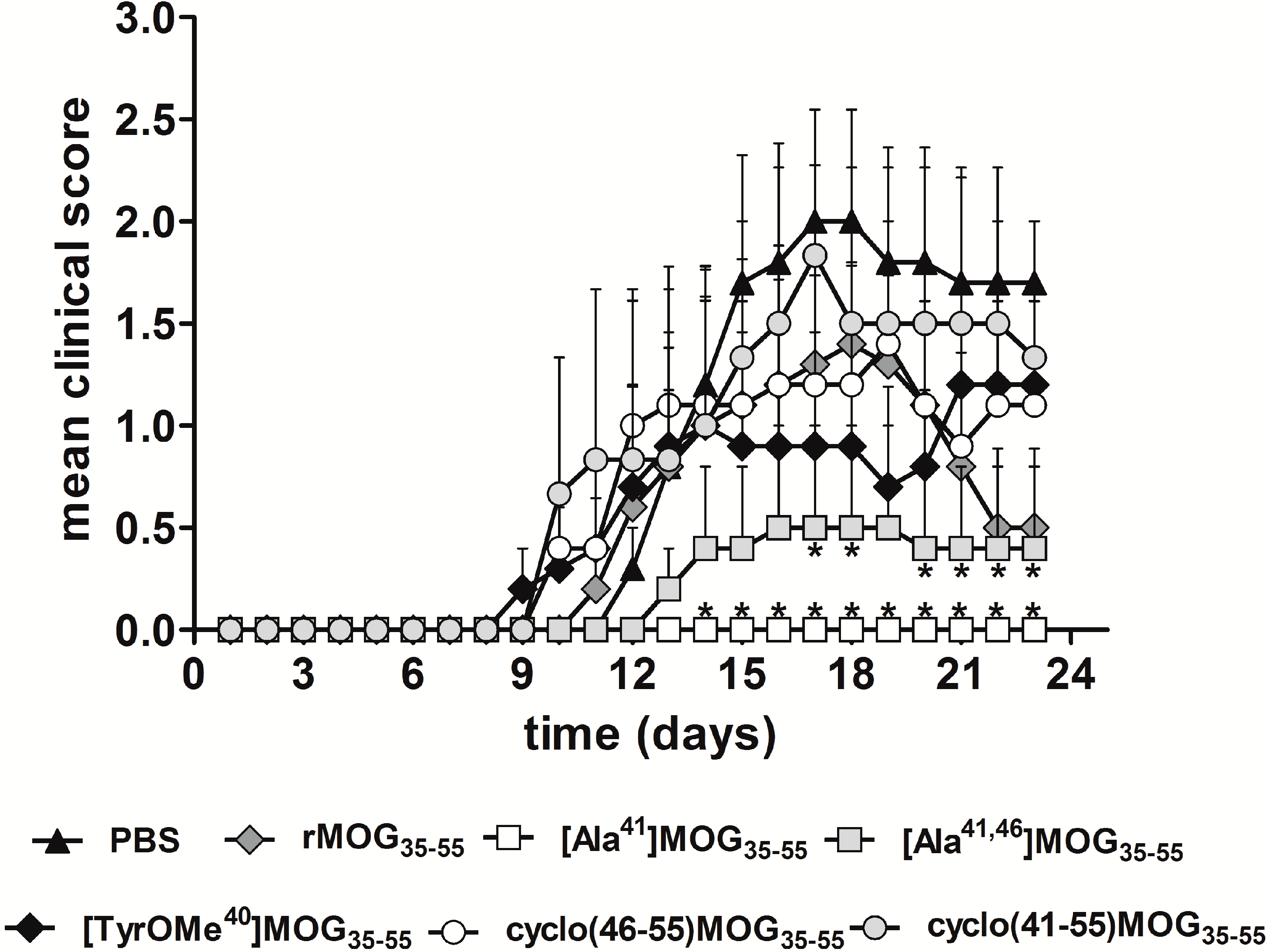
2.2. Inhibitory Activity of MOG APLs in the EAE.
3. Experimental Section
3.1. General
3.2. Synthesis
3.2.1. Conventional Solid Phase Peptide Synthesis (SPPS) of Linear Peptides
Cleavage from the Resin and Final Deprotection
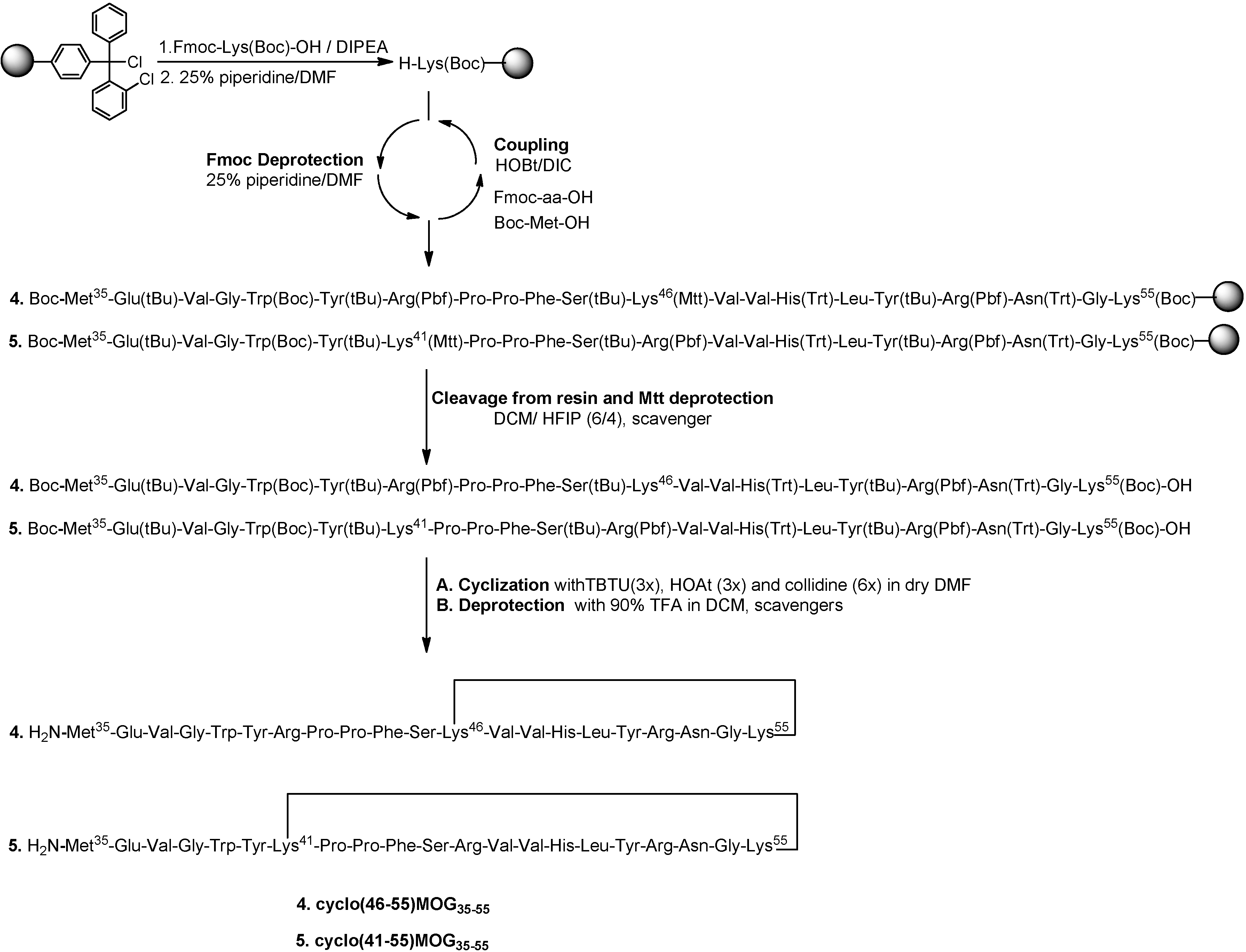
3.2.2. Synthesis of Cyclic Peptides: Cyclo(46–55)MOG35–55 and Cyclo(41–55)MOG35–55
Conventional SPPS of Precursor Protected Linear Peptides
Cleavage from the Resin and Mtt Deprotection
Cyclization of Protected Peptides and Final Deprotection
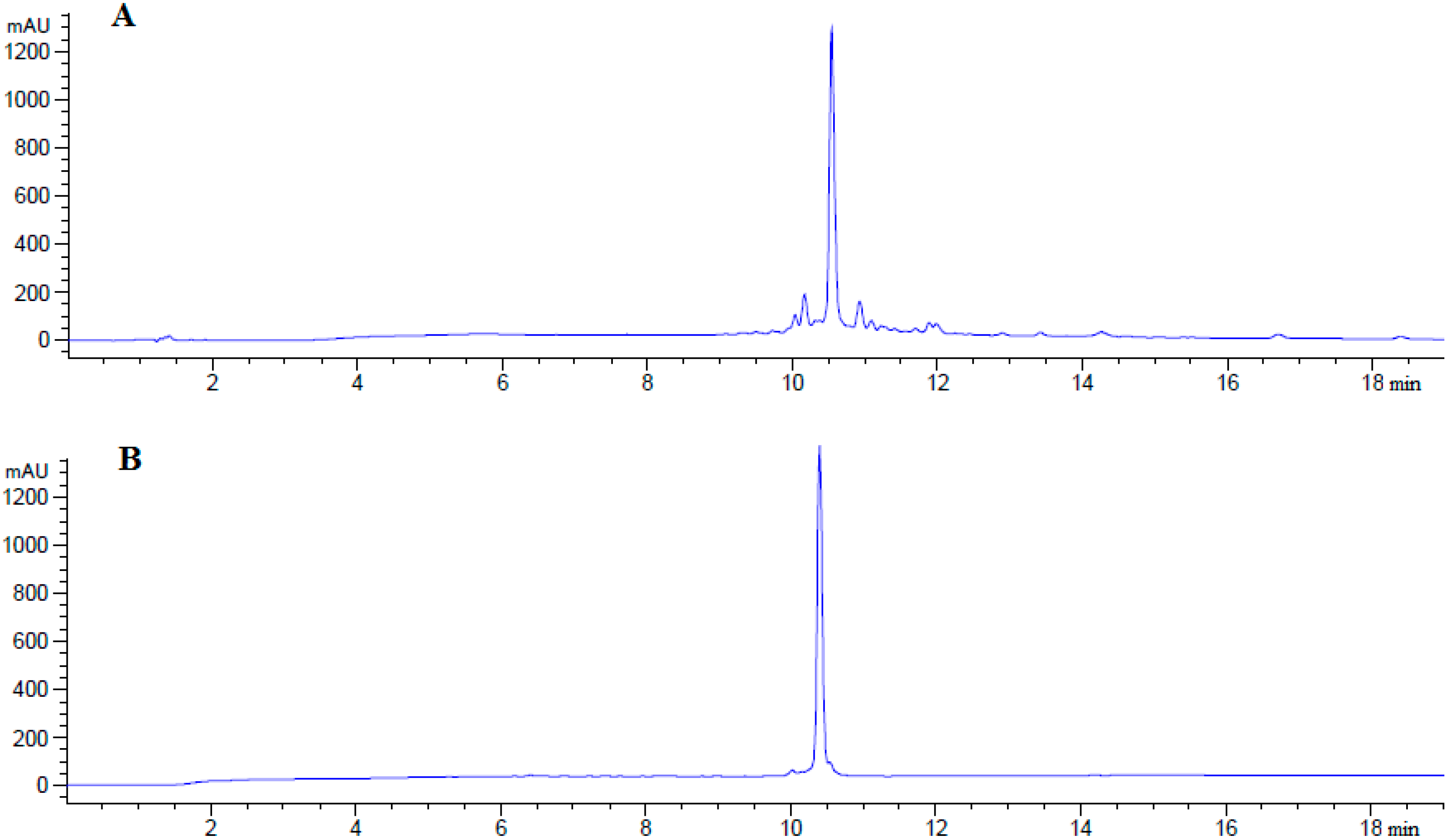
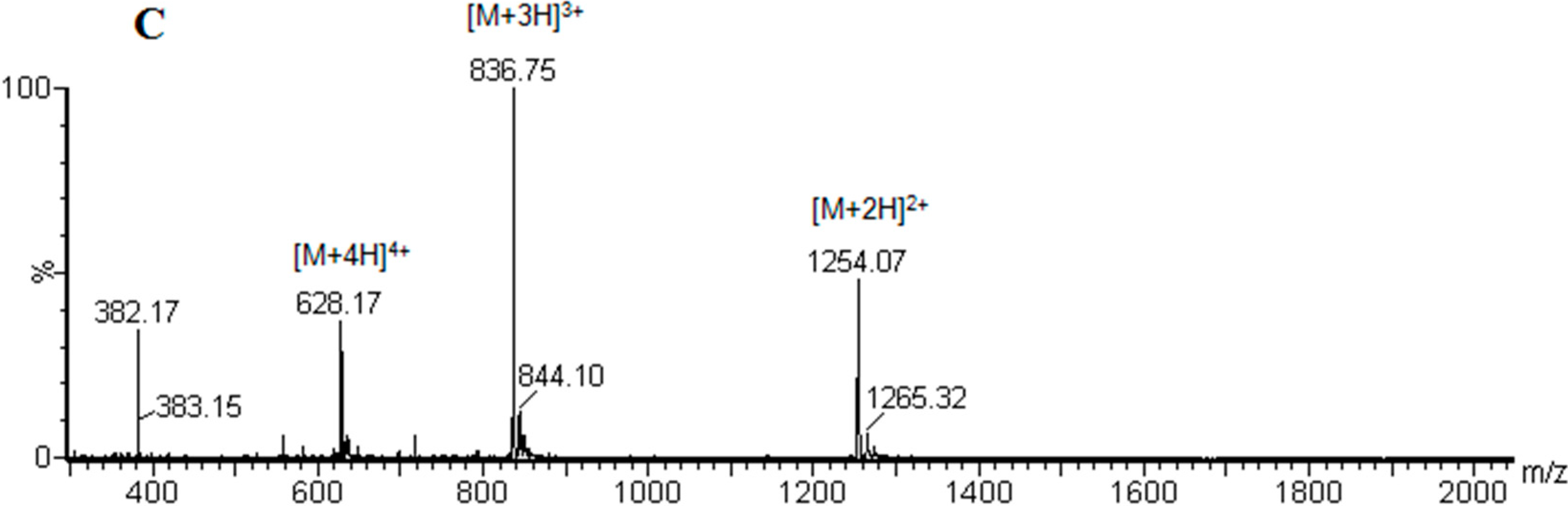
3.3. Peptide Analysis and Purification
3.4. Biological Evaluation
3.4.1. Animals and Ethics Statement
3.4.2. EAE Induction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl J. Med. 2000, 343, 938–952. [Google Scholar]
- Steinman, L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 1996, 85, 299–302. [Google Scholar]
- Mantzourani, E.D.; Mavromoustakos, T.M.; Platts, J.A.; Matsoukas, J.M.; Tselios, T. Structural requirements for binding of myelin basic protein (MBP) peptides to MHC II: Effects on immune regulation. Curr. Med. Chem. 2005, 12, 1521–1535. [Google Scholar]
- Lassmann, H. New concepts on progressive multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2007, 7, 239–244. [Google Scholar]
- Hemmer, B.; Cepok, S.; Zhou, D.; Sommer, N. Multiple sclerosis—A coordinated immune attack across the blood brain barrier. Curr. Neurovasc. Res. 2004, 1, 141–150. [Google Scholar]
- Martin, R.; McFarland, H.; McFarlin, D. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 1992, 10, 153–187. [Google Scholar]
- Martin, R.; McFarland, H.F. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit. Rev. Clin. Lab. Sci. 1995, 32, 121–182. [Google Scholar]
- Kappos, L.; Comi, G.; Panitch, H.; Oger, J.; Antel, J.; Conlon, P.; Steinman, L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat. Med. 2000, 6, 1176–1182. [Google Scholar]
- Bielekova, B.; Goodwin, B.; Richert, N.; Cortese, I.; Kondo, T.; Afshar, G.; Gran, B.; Eaton, J.; Antel, J.; Frank, J.A.; et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000, 6, 1167–1175. [Google Scholar]
- Tselios, T.; Apostolopoulos, V.; Daliani, I.; Deraos, S.; Grdadolnik, S.G.; Mavromoustakos, T.; Melachrinou, M.; Thymianou, S.; Probert, L.; Mouzaki, A.; et al. Antagonistic effects of human cyclic MBP(87–99) altered peptide ligands in experimental allergic encephalomyelitis and human T-cell proliferation. J. Med. Chem. 2002, 45, 275–283. [Google Scholar]
- Mantzourani, E.D.; Tselios, T.V.; Grdadolnik, S.G.; Platts, J.A.; Brancale, A.; Deraos, G.; Matsoukas, J.M.; Mavromoustakos, T.M. Comparison of proposed putative active conformations of linear altered peptide ligands of myelin basic protein epitope 87–99 by spectroscopic and modelling studies: The role of position 91 and 96 in T-cell receptor activation. J. Med. Chem. 2006, 49, 6683–6691. [Google Scholar]
- Mantzourani, E.; Tselios, T.; Grdadolnik, S.G.; Brancale, A.; Matsoukas, J.; Mavromoustakos, T. A putative bioactive conformation for the altered peptide ligand of myelin basic protein and inhibitor of experimental autoimmune encephalomyelitis [Arg91, Ala96]MBP87–99. J. Mol. Graph. Model. 2006, 25, 17–29. [Google Scholar]
- Deraos, G.; Chatzantoni, K.; Matsoukas, M.T.; Tselios, T.; Deraos, S.; Katsara, M.; Papathanasopoulos, P.; Vynios, D.; Apostolopoulos, V.; Mouzaki, A.; et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein MBP(87–99) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: Implications in triggering disease. J. Med. Chem. 2008, 51, 7834–7842. [Google Scholar]
- Spyranti, Z.; Dalkas, G.A.; Spyroulias, G.A.; Mantzourani, E.D.; Mavromoustakos, T.; Friligou, I.; Matsoukas, J.M.; Tselios, T.V. Putative bioactive conformations of amide linked cyclic myelin basic protein peptide analogues associated with experimental autoimmune encephalomyelitis. J. Med. Chem. 2007, 50, 6039–6047. [Google Scholar]
- Mantzourani, E.D.; Blokar, K.; Tselios, T.V.; Matsoukas, J.M.; Platts, J.A.; Mavromoustakos, T.M.; Grdadolnik, S.G. A combined NMR and molecular dynamics simulation study to determine the conformational properties of agonists and antagonists against experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2008, 16, 2171–2182. [Google Scholar]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar]
- Vergelli, M.; Hemmer, B.; Utz, U.; Vogt, A.; Kalbus, M.; Tranquill, L.; Conlon, P.; Ling, N.; Steinman, L.; McFarland, H.F.; et al. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87–99). Eur. J. Immunol. 1996, 26, 2624–2634. [Google Scholar]
- Warren, K.G.; Catz, I.; Ferenczi, L.Z.; Krantz, M.J. Intravenous synthetic peptide MBP82–98 delayed disease progression in an HLA Class II-defined cohort of patients with progressive multiple sclerosis: results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006, 13, 887–895. [Google Scholar]
- Kerlero de Rosbo, N.; Milo, R.; Lees, M.B.; Burger, D.; Bernard, C.C.; Ben-Nun, A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J. Clin. Investig. 1993, 92, 2602–2608. [Google Scholar]
- Linington, C.; Berger, T.; Perry, L.; Weerth, S.; Hinze-Selch, D.; Zhang, Y.; Lu, H.C.; Lassmann, H.; Wekerle, H. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur. J. Immunol. 1993, 23, 1364–1372. [Google Scholar]
- Amor, S.; Groome, N.; Linington, C.; Morris, M.M.; Dornmair, K.; Gardinier, M.V.; Matthieu, J.M.; Baker, D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 1994, 153, 4349–4356. [Google Scholar]
- Ichikawa, M.; Johns, T.G.; Liu, J.; Bernard, C.C. Analysis of the fine B cell specificity during the chronic/relapsing course of a multiple sclerosis-like disease in Lewis rats injected with the encephalitogenic myelin oligodendrocyte glycoprotein peptide 35–55. J. Immunol. 1996, 157, 919–926. [Google Scholar]
- Zhou, D.; Srivastava, R.; Nessler, S.; Grummel, V.; Sommer, N.; Brück, W.; Hartung, H.P.; Stadelmann, C.; Hemmer, B. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2006, 12, 103, 19057–19062. [Google Scholar]
- Kerlero de Rosbo, N.; Mendel, I.; Ben-Nun, A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur. J. Med. Chem. 1995, 25, 985–993. [Google Scholar]
- Mendel, I.; Kerlero de Rosbo, N.; Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur. J. Med. Chem. 1995, 25, 1951–1959. [Google Scholar]
- Slavin, A.; Ewing, C.; Liu, J.; Ichikawa, M.; Slavin, J.; Bernard, C.C. Induction of a multiple sclerosis-like disease in mice with an immunodominant epitope of myelin oligodendrocyte glycoprotein. Autoimmunity 1998, 28, 109–120. [Google Scholar]
- Forsthuber, T.G.; Shive, C.L.; Wienhold, W.; de Graaf, K.; Spack, E.G.; Sublett, R.; Melms, A.; Kort, J.; Racke, M.K.; Weissert, R. T Cell Epitopes of Human Myelin Oligodendrocyte Glycoprotein Identified in HLA-DR4 (DRB1*0401) Transgenic Mice Are Encephalitogenic and Are Presented by Human B Cells. J. Immunol. 2001, 167, 7119–7125. [Google Scholar]
- Katsara, M.; Tselios, T.; Deraos, S.; Deraos, G.; Matsoukas, M.T.; Lazoura, E.; Matsoukas, J.; Apostolopoulos, V. Round and round we go: Cyclic peptides in disease. Curr. Med. Chem. 2006, 13, 2221–2232. [Google Scholar]
- Namjoshi, S.; Benson, H.A. Cyclic peptides as potential therapeutic agents for skin disorders. Biopolymers 2010, 94, 673–680. [Google Scholar]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar]
- Tselios, T.; Daliani, I.; Deraos, S.; Thymianou, S.; Matsoukas, E.; Troganis, A.; Gerothanassis, I.; Mouzaki, A.; Mavromoustakos, T.; Probert, L.; et al. Treatment of Experimental Allergic Encephalomyelitis (EAE) by a Rationally Designed Cyclic Analogue of Myelin Basic Protein (MBP) Epitope 72–85. Bioorg. Med. Chem. Lett. 2000, 10, 2713–2717. [Google Scholar]
- Oliver, A.R.; Lyon, G.M.; Ruddle, N.H. Mechanisms in C57BL/6 Mice Autoimmune Encephalomyelitis by Different Glycoproteins Induce Experimental Rat and Human Myelin Oligodendrocyte. J. Immunol. 2003, 171, 462–468. [Google Scholar]
- Barlos, K.; Gatos, D.; Kapolos, S.; Papaphotiu, G.; Schafer, W.; Wenqing, Y. Esterification of partially protected peptide-fragmentswith resins-utilization of 2-chlorotritylchloride for synthesis of Leu-15-gastrin-I. Tetrahedron Lett. 1989, 30, 3947–3950. [Google Scholar]
- Barlos, K.; Gatos, D.; Schafer, W. Synthesis of prothymosin α (ProTα)—A protein consisting of 109 amino acid residues. Angew. Chem. Int. Ed. 1991, 30, 590–593. [Google Scholar]
- Friligou, I.; Papadimitriou, E.; Gatos, D.; Matsoukas, J.; Tselios, T. Microwave-assisted solid-phase peptide synthesis of the 60–110 domain of human pleiotrophinon 2-chlorotrityl resin. Amino Acids 2011, 40, 1431–1440. [Google Scholar]
- Friligou, I.; Rizzolo, F.; Nuti, F.; Tselios, T.; Evangelidou, M.; Emmanouil, M.; Karamita, M.; Matsoukas, J.; Chelli, M.; Rovero, P.; et al. Divergent and convergent synthesis of polymannosylated dibranched antigenic peptide of the immunodominant epitope MBP(83–99). Bioorg. Med. Chem. 2013, 21, 6718–6725. [Google Scholar]
- Petersen, T.R.; Bettelli, E.; Sidney, J.; Sette, A.; Kuchroo, V.; Bäckström, B.T. Characterization of MHC- and TCR-binding residues of the myelin oligodendrocyte glycoprotein 38–51 peptide. Eur. J. Immunol. 2004, 34, 165–173. [Google Scholar]
- Krishnamoorthy, G.; Saxena, A.; Mars, L.T.; Domingues, H.S.; Mentele, R.; Ben-Nun, A.; Lassmann, H.; Dornmair, K.; Kurschus, F.C.; Liblau, R.S.; et al. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat. Med. 2009, 15, 626–632. [Google Scholar]
- Plebanski, M.; Flanagan, L.; Lee, A.; Reece, H.; Hart, K.; Gelder, C.; Gillespie, G.; Pinder, M.; Hill, V. Interleukin 10-mediated immunosuppression by a variant CD4 T Cell epitope of plasmodium falciparum. Immunity 1999, 10, 651–660. [Google Scholar]
- Plebanski, M.; Lee, A.; Hannan, M.; Flanagan, L.; Gilbert, C.; Gravenor, B.; Hill, V. Altered peptide ligands narrow the repertoire of cellular immune responses by interfering with T-cell priming. Nat. Med. 1999, 5, 565–571. [Google Scholar]
- Young, A.; Lowe, D.; Booth, S.; Whitters, J.; Nicholson, L.; Kuchroo, K.; Collins, M. IL-4, IL-10, IL-13, and TGF-β from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J. Immunol. 2000, 164, 3563–3572. [Google Scholar]
- Tseveleki, V.; Bauer, J.; Taoufik, E.; Ruan, C.; Leondiadis, L.; Haralambous, S.; Lassmann, H.; Probert, L. Cellular FLIP (long isoform) overexpression in T cells drives Th2 effector responses and promotes immunoregulation in experimental autoimmune encephalomyelitis. J. Immunol. 2004, 173, 6619–6626. [Google Scholar]
- Adelmann, M.; Wood, J.; Benzel, I.; Fiori, P.; Lassmann, H.; Matthieu, J.M.; Gardinier, M.V.; Dornmair, K.; Linington, C. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J. Neuroimmunol. 1995, 63, 17–27. [Google Scholar]
- Juedes, A.E.; Hjelmström, P.; Bergman, C.M.; Neild, A.L.; Ruddle, N.H. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyteglycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 2000, 164, 419–426. [Google Scholar]
- Sample Availability: Samples of the final linear and cyclic peptides are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tselios, T.; Aggelidakis, M.; Tapeinou, A.; Tseveleki, V.; Kanistras, I.; Gatos, D.; Matsoukas, J. Rational Design and Synthesis of Altered Peptide Ligands based on Human Myelin Oligodendrocyte Glycoprotein 35–55 Epitope: Inhibition of Chronic Experimental Autoimmune Encephalomyelitis in Mice. Molecules 2014, 19, 17968-17984. https://doi.org/10.3390/molecules191117968
Tselios T, Aggelidakis M, Tapeinou A, Tseveleki V, Kanistras I, Gatos D, Matsoukas J. Rational Design and Synthesis of Altered Peptide Ligands based on Human Myelin Oligodendrocyte Glycoprotein 35–55 Epitope: Inhibition of Chronic Experimental Autoimmune Encephalomyelitis in Mice. Molecules. 2014; 19(11):17968-17984. https://doi.org/10.3390/molecules191117968
Chicago/Turabian StyleTselios, Theodore, Mihalis Aggelidakis, Anthi Tapeinou, Vivian Tseveleki, Ioannis Kanistras, Dimitrios Gatos, and John Matsoukas. 2014. "Rational Design and Synthesis of Altered Peptide Ligands based on Human Myelin Oligodendrocyte Glycoprotein 35–55 Epitope: Inhibition of Chronic Experimental Autoimmune Encephalomyelitis in Mice" Molecules 19, no. 11: 17968-17984. https://doi.org/10.3390/molecules191117968