Hepatoprotective Effect of the Aqueous Extract of Simarouba amara Aublet (Simaroubaceae) Stem Bark against Carbon Tetrachloride (CCl4)-Induced Hepatic Damage in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Analyzes
2.1.1. Thin Layer Chromatography
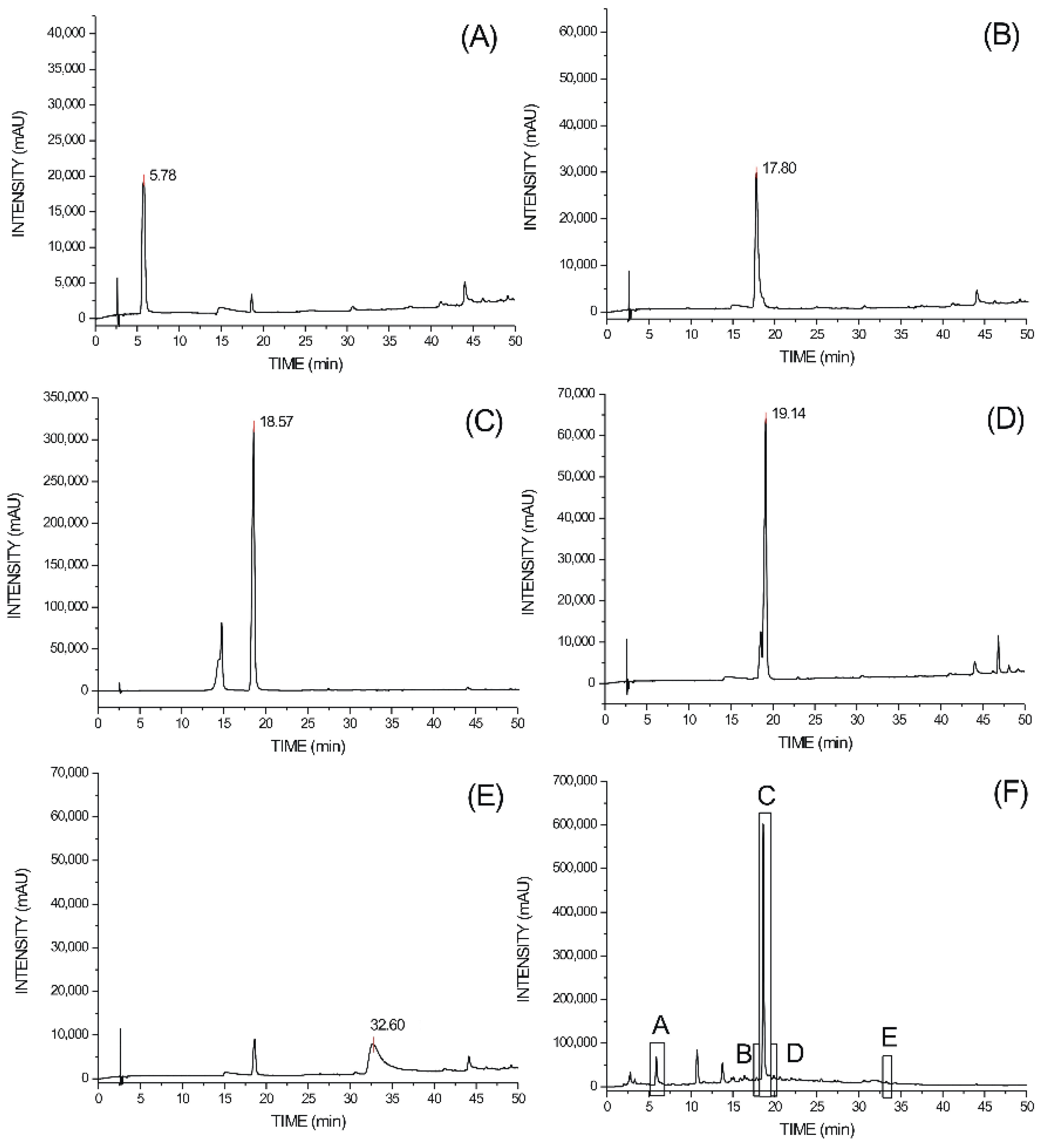
2.1.2. High Performance Liquid Chromatography
2.2. Evaluation of Lipid Peroxidation, Catalase and Superoxide Dismutase Levels
| Groups | MDA (nmol/mg Protein) | CAT (mU/mg Protein) | SOD (U/mg Protein) |
|---|---|---|---|
| Control | 5.94 ± 0.88 | 0.33 ± 0.03 | 11.96 ± 0.06 |
| Vehicle | 3.88 ± 0.44 a | 0.46 ± 0.04 b | 11.89 ± 0.17 b |
| Legalon® | 3.19 ± 0.36 a | 0.40 ± 0.02 b | 11.57 ± 0.62 b |
| SAAE 100 + CCl4 | 3.42 ± 0.37 a | 0.45 ± 0.05 b | 12.22 ± 0.13 b |
| SAAE 250 + CCl4 | 2.69 ± 0.42 a | 0.92 ± 0.12 a | 11.77 ± 0.21 b |
| SAAE 500 + CCl4 | 3.56 ± 0.55 a | 0.83 ± 0.02 a | 12.17 ± 0.34 b |
2.3. Biochemical Parameters
| Groups | AST (U/L) | ALT (U/L) | AF (U/L) | GGT (U/L) | TB (mg/dL) | DB (mg/dL) | IB (mg/dL) | LDH (U/L) |
|---|---|---|---|---|---|---|---|---|
| Control | 2001.0 ± 7.4 | 2478 ± 6.5 | 125.6 ± 6.4 | 2.2 ± 0.6 | 0.42 ± 0.07 | 0.26 ± 0.08 | 0.13 ± 0.03 | 2560.0 ± 8.8 |
| Vehicle | 85.5 ± 4.8 a | 43.7 ± 2.3 a | 96.8 ± 5.5 | 0.2 ± 0.2 a | 0.10 ± 0.00 a | 0.08 ± 0.01 | 0.01 ± 0.01 a | 328.6 ± 3.6 a |
| Legalon® | 213.7 ± 8.7 a | 120.8 ± 5.0 a | 100.0 ± 8.3 | 1.5 ± 0.6 | 0.25 ± 0.15 a | 0.20 ± 0.10 | 0.05 ± 0.05 a | 421.5 ± 5.5 a |
| SAAE 100 + CCl4 | 501.8 ± 9.3 a | 576.5 ± 6.3 a | 92.7 ± 8.7 | 0.8 ± 0.3 | 0.10 ± 0.00 a | 0.10 ± 0.00 | 0.00 ± 0.00 a | 599.5 ± 16.4 a |
| SAAE 250 + CCl4 | 419.7 ± 7.7 a | 541.0 ± 14.2 a | 91.2 ± 7.5 | 0.8 ± 0.4 | 0.10 ± 0.00 a | 0.10 ± 0.00 | 0.00 ± 0.00 a | 462.8 ± 7.9 a |
| SAAE 500 + CCl4 | 252.8 ± 5.4 a | 220.6 ± 6.9 a | 86.2 ± 6.5 | 0.3 ± 0.2 a | 0.12 ± 0.00 a | 0.10 ± 0.00 | 0.02 ± 0.00 a | 337.8 ± 2.6 a |
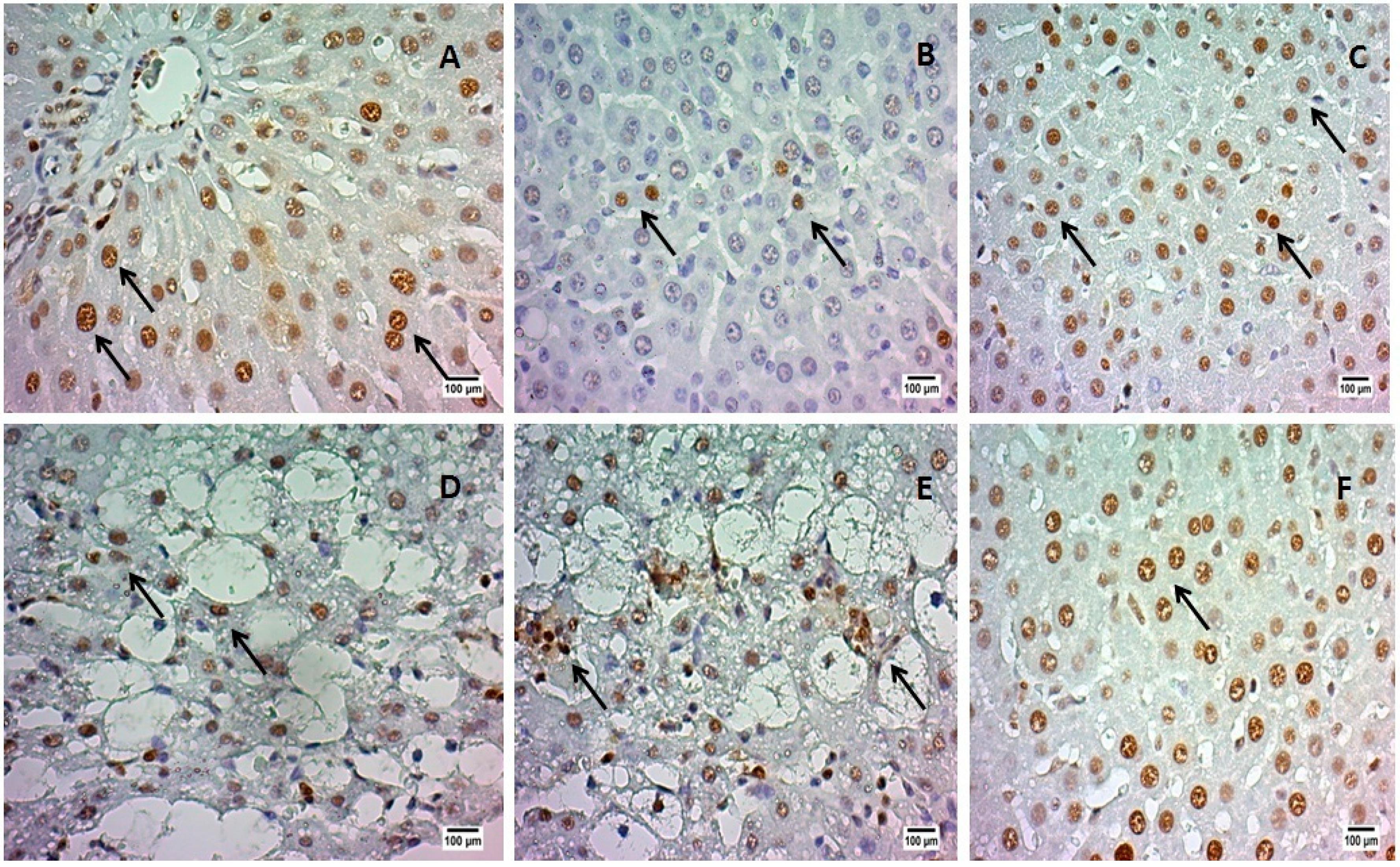
2.4. Proliferating Cell Nuclear Antigen (PCNA) Analysis
3. Experimental Section
3.1. Chemical and Reagents
3.2. Material Plant
3.3. Extract Preparation
3.4. Chromatographic Analyzes
3.4.1. Thin Layer Chromatography
3.4.2. High Performance Liquid Chromatography
3.5. Animals
3.6. Treatment
3.7. Liver Analyzes
3.7.1. Liver Homogenate
3.7.2. Lipid Peroxidation Assay
3.7.3. Superoxide Dismutase (SOD) Assay
3.7.4. Catalase (CAT) Assay
3.7.5. Proliferating Cell Nuclear Antigen (PCNA) Imunohistochemical
3.8. Biochemical Parameters
3.9. Statiscal Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arriaga, A.M.C.; Mesquita, A.C.; Pouliquen, Y.B.M.; Mesquita, A.C.; Lima, R.A.; Cavalcante, S.H.; Carvalho, M.G.; Siqueira, J.A.; Alegrio, L.V.; Braz-filho, R. Chemical constituents of Simarouba versicolor. An. Acad. Bras. Ciênc. 2002, 73, 415–424. [Google Scholar]
- Espinal, T.; Sigifredo, L. Arboles de Antioquia; Universidad Nacional de Colombia: Medellín, Colombia, 1986; pp. 200–222. [Google Scholar]
- Bonté, F.; Barret, P.; Pinguet, P.; Dusser, I.; Dumas, M.; Meybec, A. Simarouba amara extract increases human skin keratinocyte differentiation. J. Ethnopharmacol. 1996, 53, 65–74. [Google Scholar] [CrossRef]
- Botsaris, A.S. Plants used traditionally to treat malaria in Brazil: The archives of Flora Medicinal. J. Ethnobiol. Ethnopharmacol. 2007, 18, 1–8. [Google Scholar]
- O’Neill, M.J.; Bray, D.H.; Boardman, P.; Wright, C.W.; Phillipson, J.D.; Warhurst, D.C.; Gupta, M.P.; Correya, M.; Solis, P. Plants as sources of antimalarial drugs, Part 6: Activities of Simarouba. amara fruits. J. Ethnopharmacol. 1988, 22, 183–190. [Google Scholar] [CrossRef]
- Kaner, E.; Newbury-Birch, D.; Avery, L.; Jackson, K.; Brown, N.; Mason, H. A Rapid Review of Liver Disease Epidemiology, Treatment and Service Provision in England; Institute of Health and Society: Newcastle, UK, 2007; pp. 200–212. [Google Scholar]
- Bansal, A.K.; Bansal, M.; Soni, G.; Bhatnagar, D. Protective role of vitamin E pre-treatment N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005, 156, 101–111. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Tsai, C.F.; Chang, W.H.; Ho, Y.C.; Chen, W.K.; Lu, F.J. Protective effects of Dunaliella salina—A carotenoids-rich alga, against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2008, 46, 3311–3317. [Google Scholar] [CrossRef]
- Cengiz, N.; Kavak, S.; Guzel, A.; Ozbek, H.; Bektas, H.; Him, A.; Erdogan, E.; Balahoroglu, R. Investigation of the hepatoprotective effects of Sesame (Sesamum indicum L.) in carbon tetrachloride-induced liver toxicity. J. Membr. Biol. 2013, 246, 1–6. [Google Scholar] [CrossRef]
- Polonsky, J.; Varon, Z.; Jacquemin, H.; Pettit, G.R. The isolation and structure of 13,18-dehydroglaucarubinone, a new antineoplastic quassinoid from Simarouba amara. Experientia 1978, 32, 1122–1123. [Google Scholar] [CrossRef]
- Grosvenor, S.N.J.; Mascoll, K.; McLean, S.; Reynolds, W.S.; Tinto, W.S. Tirucallane, apotirucallane, and octanorapotirucallane triterpenes of Simarouba amara. J. Nat. Prod. 2006, 69, 1315–1318. [Google Scholar] [CrossRef]
- Coimbra, R. Manual de Fitoterapia, 2nd ed.; Cejup: Belém, Brazil, 1994; pp. 300–335. [Google Scholar]
- Hanzlik, R.P. Reactivity and toxicity among halogenated methanes and related compounds. Biochem. Pharmacol. 1981, 30, 3027–3030. [Google Scholar] [CrossRef]
- Slater, T.F. Biochemical implications of current studies on vitamin E. Ann. N. Y. Acad. Sci. 1982, 393, 496–500. [Google Scholar] [CrossRef]
- Brattin, W.J.; Glende, E.A.; Recknagel, R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985, 1, 27–38. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Khan, M.R.; Ahmed, D. Protective effects of Digera. muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol. 2009, 47, 1393–1399. [Google Scholar] [CrossRef]
- Naik, S.R.; Panda, V.S. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Intern. 2007, 27, 393–399. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Ahn, T.-H.; Lee, J.C.; Moon, C.J.; Kim, S.H.; Jun, W.; Park, S.C.; Kim, H.C.; Kim, J.-C. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 2008, 46, 380–387. [Google Scholar] [CrossRef]
- Lima, R.K.; Cardoso, M.G.; Andrade, M.A.; Guimaraes, P.L.; Batista, L.R.; Nelson, D.L. Bactericidal and antioxidant activity of essential oils from Myristica. fragrans Houtt and Salvia microphylla H.B.K. J. Am. Oil Chem. Soc. 2012, 89, 523–528. [Google Scholar] [CrossRef]
- Singh, N.; Kamath, V.; Narasimhamurthy, K.; Rajini, P.S. Protective effects of potato peel extract against carbon tetrachloride-induced liver injury in rats. Environ. Toxicol. Pharmacol. 2008, 26, 241–146. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Shah, N.A. Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: A randomized controlled trial. BMC Complement. Altern. Med. 2012, 12, 90–97. [Google Scholar] [CrossRef]
- Arisawa, E.A.L.; Moraes, E.; Rocha, R.F.; Almeida, J.D. Marcadores biológicos: PCNA e Ki-67. Breve revisão. Pós-Grad. Rev. Fac. Odontol. 1999, 2, 54–60. [Google Scholar]
- Majumdar, S.; Moudgal, R.P. Effect of tannic acid on activities of certain digestive enzymesand alkaline phosphatase in intestine and glucose absorption in adult chickens. J. Appl. Anim. Res. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis, 2nd ed.; Springer: New York, NY, USA, 1996; pp. 310–354. [Google Scholar]
- Simile, M.M.; Bani, S.; Angioni, E.; Carta, G.; Feo, F. 5'-Methylthioadensine administration prevents lipid peroxidation and fibrinogenesis induced in rat liver by carbon-tetrachloride intoxication. J. Hepatol. 2001, 34, 386–394. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1984, 52, 302–310. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Vasconcelos, C.F.B.; Maranhão, H.M.L.; Batista, T.M.; Carneiro, E.M.; Ferreira, F.; Costa, J.; Soares, L.A.L.; Sá, M.D.C.; Souza, T.P.; Wanderley, A.G. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea Martius bark extract on streptozotocin-induced diabetes in Wistar rats. J. Ethnopharmacol. 2011, 137, 1533–1541. [Google Scholar] [CrossRef]
- Sample Availability: Samples of Simarouba amara stem bark are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranhão, H.M.L.; Vasconcelos, C.F.B.; Rolim, L.A.; Neto, P.J.R.; Neto, J.D.C.S.; Filho, R.C.d.S.; Fernandes, M.P.; Costa-Silva, J.H.; Araújo, A.V.; Wanderley, A.G. Hepatoprotective Effect of the Aqueous Extract of Simarouba amara Aublet (Simaroubaceae) Stem Bark against Carbon Tetrachloride (CCl4)-Induced Hepatic Damage in Rats. Molecules 2014, 19, 17735-17746. https://doi.org/10.3390/molecules191117735
Maranhão HML, Vasconcelos CFB, Rolim LA, Neto PJR, Neto JDCS, Filho RCdS, Fernandes MP, Costa-Silva JH, Araújo AV, Wanderley AG. Hepatoprotective Effect of the Aqueous Extract of Simarouba amara Aublet (Simaroubaceae) Stem Bark against Carbon Tetrachloride (CCl4)-Induced Hepatic Damage in Rats. Molecules. 2014; 19(11):17735-17746. https://doi.org/10.3390/molecules191117735
Chicago/Turabian StyleMaranhão, Hélida M. L., Carlos F. B. Vasconcelos, Larissa A. Rolim, Pedro J. Rolim Neto, Jacinto Da C. Silva Neto, Reginaldo C. da Silva Filho, Mariana P. Fernandes, João H. Costa-Silva, Alice V. Araújo, and Almir G. Wanderley. 2014. "Hepatoprotective Effect of the Aqueous Extract of Simarouba amara Aublet (Simaroubaceae) Stem Bark against Carbon Tetrachloride (CCl4)-Induced Hepatic Damage in Rats" Molecules 19, no. 11: 17735-17746. https://doi.org/10.3390/molecules191117735




