Use of Carbon Nanotubes (CNTs) with Polymers in Solar Cells
Abstract
:1. Introduction
2. Overview of Carbon Nanotubes
2.1. Structure and Classification of CNTs
2.2. Single-Walled Carbon Nanotubes (SWCNTs)
2.3. Multi-Walled Carbon Nanotubes (MWCNTs)
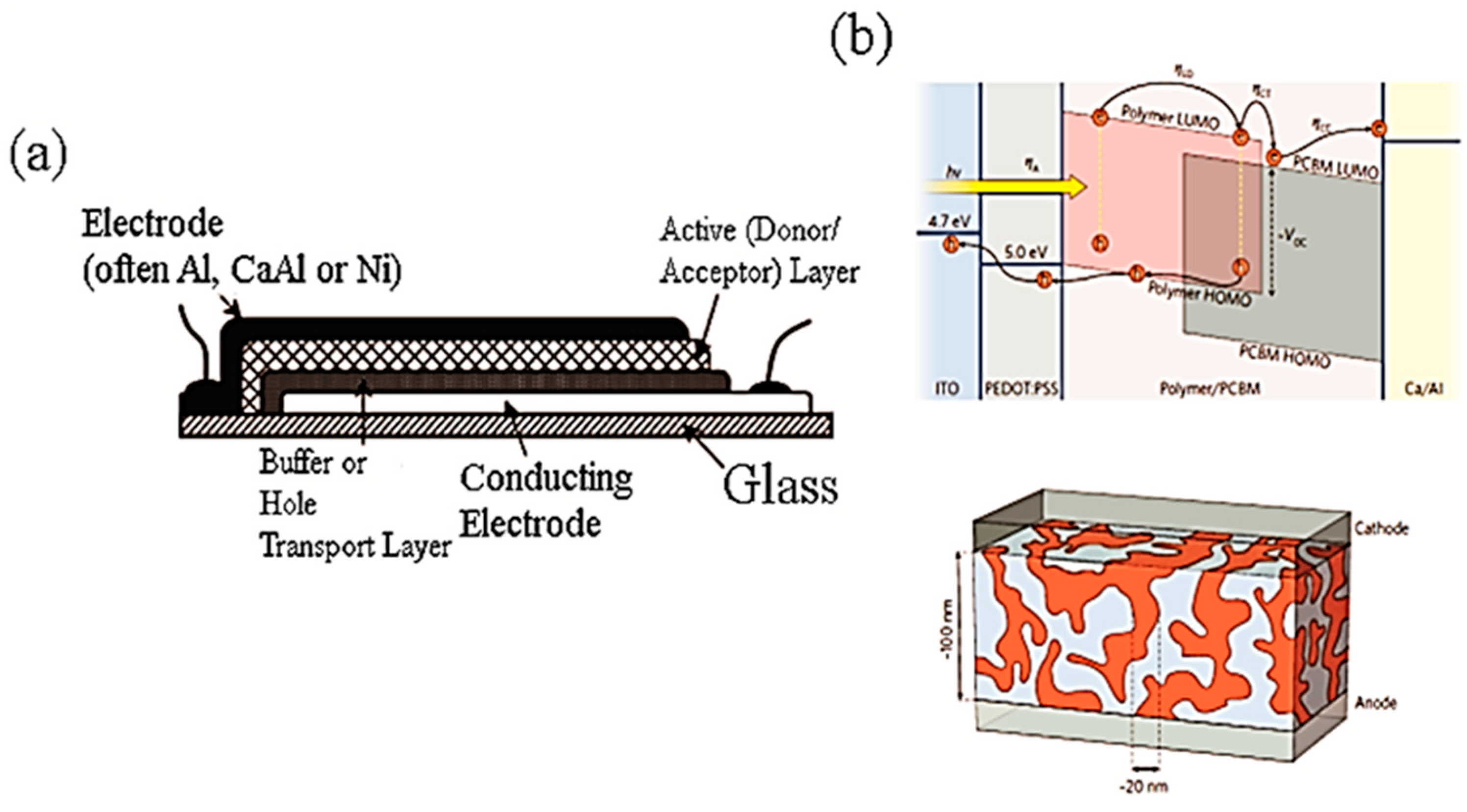
3. CNTs in Polymer Solar Cells
| Device Structure | JSC (mA/cm2) | Voc (V) | FF | η (%) | Spectrum (mW/cm2) | Ref. |
|---|---|---|---|---|---|---|
| Glass/SWCNT/PEDOT:PSS/P3HT:PCBM/Ga;In | 6.50 | 0.50 | 0.30 | 0.99 | -/100 | [26] |
| Glass/ITO/P3OT/P3OT:SWCNT/Al | 0.12 | 0.75 | 0.40 | 0.04 | AM 1.5/100 | [31] |
| Glass/ITO/PEDOT:PSS/PTEBS:MWCNT/C60/Al | 1.52 | 0.57 | 0.62 | 0.55 | AM 1.5/100 | [32] |
| Glass/SWCNT/PEDOT:PSS/P3HT:PCBM/Ca/Al | 13.78 | 0.57 | 0.53 | 4.13 | AM 1.5/100 | [33] |
| FTO/PBT/POT/SWCNT-TIOPH/Ca/Al | 1.81 | 1.48 | AM1.5/155 | [34] | ||
| Glass/SWCNT/P3HT:PCBM/Al | 4.46 | 0.36 | 0.38 | 0.61 | AM 1.5/100 | [35] |
| Glass/SWCNT(H2O:SDS)/PEDOT:PSS/P3HT:PCBM/LiF/Al | 7.30 | 0.59 | 0.46 | 2.2 | AM 1.5/100 | [36] |
| PET/SWCNT/PEDOT:PSS/P3HT:PCBM/Al | 7.80 | 0.61 | 0.52 | 2.5 | AM 1.5G/100 | [37] |
| PET/SWCNT/ZnO-nw/P3HT/Au | - | - | - | ~0.60 | AM 1.5G/100 | [38] |
| Glass/ITO/MWCNT/P3HT:PCBM/LiF/Al | 4.00 | 0.50 | 0.47 | 0.93 | AM 1.5/100 | [39] |
| Glass/ITO/PEDOT:PSS/P3HT:PCBM:MWCNT/LiF/Al | 9.33 | 0.57 | 0.38 | 2.00 | AM 1.5/100 | [40] |
| Glass/ITO/PEDOT:PSS/P3HT:C60:SWCNT/LiF/Al | 2.69 | 0.54 | 0.49 | 0.75 | AM 1.5/95 | [41] |
| Glass/ITO/PEDOT:PSS/P3HT:SWCNT/Al | 1.93 | 0.58 | 0.42 | 0.52 | -/70 | [42] |
| Glass/ITO/PEDOT:PSS/P3HT:PCBM:SWCNT/Al | 4.95 | 0.55 | 0.52 | 1.40 | AM 1.5/100 | [43] |
| Glass/ITO/PEDOT:PSS/QTF12:PCBM:DWCNT/LiF/Al | 2.37 | 0.56 | 0.37 | 0.50 | AM 1.5/100 | [44] |
| Glass/ITO/MWCNT/P3HT:PCBM/LiF/Al | 7.30 | 0.61 | 0.62 | 2.70 | AM 1.5/100 | [45] |
| Glass/ITO/ODA-SWCNT:P3HT:PC70BM/BCP/Al | 7.66 | 0.52 | 0.44 | 1.76 | AM 1.5/100 | [46] |
| Glass/FTO/MWCNTs/MoO3/P3HT:PCBM/Ca/Al | 8.88 | 0.51 | 0.46 | 2.1 | AM 1.5/100 | [47] |
| Glass/ITO P3HT:PCBM:SWCNTs/Al | 11.46 | 0.57 | 0.46 | 3.02 | AM 1.5/100 | [48] |
| Glass/ITO/PEDOT:PSS/f-MWCNT/Al | 11.15 | 0.50 | 0.30 | 1.65 | AM 1.5/100 | [49] |
| Glass/ITO/HTM/PTM10:PTM21-CNT:PCBM/LiF/Al | 0.45 | 0.88 | 0.38 | 0.15 | AM 1.5/100 | [50] |
3.1. CNTs as a Hole Extraction Layer or the Transparent Conducting Electrode

3.2. CNTs in the Active Layer
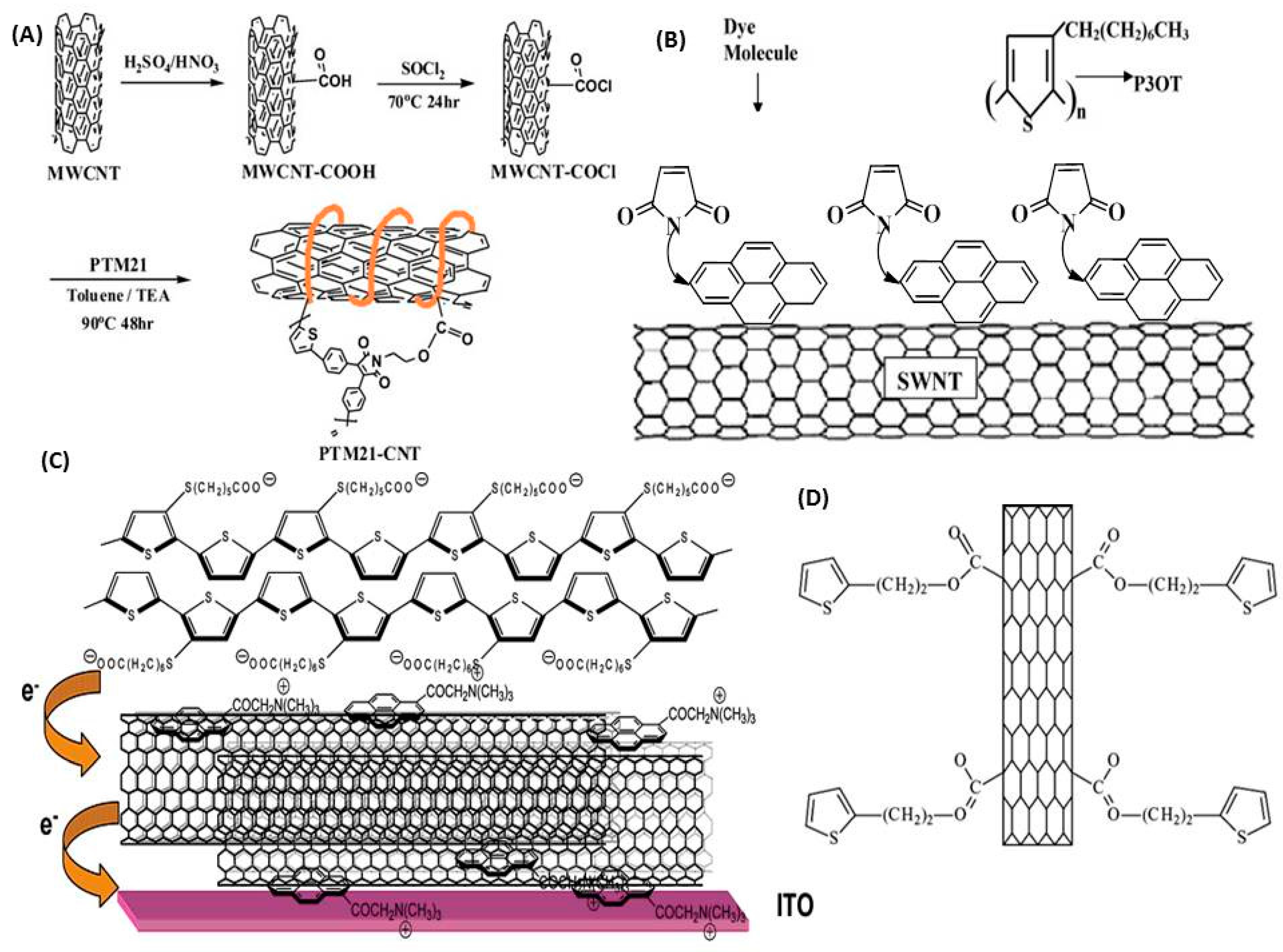
3.3. General CNT Considerations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Green, M.A.; Emery, K.; Hishikawa, Y.; Dunlop, E.D. Solar cell efficiency tables (version 39). Progress Photovolt. Res. Appl. 2012, 20, 12–20. [Google Scholar]
- Jiang, M.; Yan, X. Cu2ZnSnS4 thin film solar cells: Present status and future prospects. In Solar Cells—Research and Application Perspectives; Morales-Acevedo, A., Ed.; Intech: Rijeka, Croatia, 2013. [Google Scholar]
- Kabir, M.I.; Ibarahim, Z.; Sopian, K.; Amin, N. A review on progress of amorphous and microcrystalline silicon thin-film solar cells. Recent Pat. Electr. Eng. 2011, 4, 50–62. [Google Scholar]
- Jackson, P.; Hariskos, D.; Lotter, E.; Paetel, S.; Wuerz, R.; Menner, R.; Wischmann, W.; Powalla, M. New world record efficiency for Cu(In,Ga)Se2 thin-film solar cells beyond 20%. Prog. Photovolt. Res. Appl. 2011, 19, 894–897. [Google Scholar]
- Kranz, L.; Gretener, C.; Perrenoud, J.; Schmitt, R.; Pianezzi, F.; la Mattina, F.; Blösch, P.; Cheah, E.; Chirilă, A.; Fella, C.M.; et al. Doping of polycrystalline CdTe for high-efficiency solar cells on flexible metal foil. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Zhou, H.; Hsu, W.-C.; Duan, H.-S.; Bob, B.; Yang, W.; Song, T.-B.; Hsu, C.-J.; Yang, Y. Czts nanocrystals: A promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 2013, 6, 2822–2838. [Google Scholar]
- Mutolo, K.L.; Mayo, E.I.; Rand, B.P.; Forrest, S.R.; Thompson, M.E. Enhanced Open-Circuit Voltage in Subphthalocyanine/C60 Organic Photovoltaic Cells. J. Am. Chem. Soc. 2006, 128, 8108–8109. [Google Scholar]
- Zhokhavets, U.; Erb, T.; Gobsch, G.; Al-Ibrahim, M.; Ambacher, O. Relation between absorption and crystallinity of poly(3-hexylthiophene)/fullerene films for plastic solar cells. Chem. Phys. Lett. 2006, 418, 347–350. [Google Scholar]
- Katz, E.A.; Faiman, D.; Tuladhar, S.M. Temperature dependence for the photovoltaic device parameters of polymer-fullerene solar cells under operating conditions. J. Appl. Phys. 2011, 90, 5343–5350. [Google Scholar]
- Wright, M.; Uddin, A. Organic—Inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar]
- Yan, J.; Uddin, M.J.; Dickens, T.J.; Okoli, O.I. Carbon nanotubes (CNTs) enrich the solar cells. Sol. Energy 2013, 96, 239–252. [Google Scholar]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar]
- Wang, C.; Guo, Z.-X.; Fu, S.; Wu, W.; Zhu, D. Polymers containing fullerene or carbon nanotube structures. Prog. Polym. Sci. 2004, 29, 1079–1141. [Google Scholar]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A new silicon p-n junction photocell for converting solar radiation into electrical power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar]
- Bosi, M.; Pelosi, C. The potential of iii-v semiconductors as terrestrial photovoltaic devices. Prog. Photovolt. Res. Appl. 2007, 15, 51–68. [Google Scholar]
- Hamann, T.W.; Jensen, R.A.; Martinson, A.B.F.; van Ryswyk, H.; Hupp, J.T. Advancing beyond current generation dye-sensitized solar cells. Energy Environ. Sci. 2008, 1, 66–78. [Google Scholar]
- Wöhrle, D.; Meissner, D. Organic solar cells. Adv. Mater. 1991, 3, 129–138. [Google Scholar]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photon 2012, 6, 153–161. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gunko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar]
- See, C.H.; Harris, A.T. A review of carbon nanotube synthesis via fluidized-bed chemical vapor deposition. Ind. Eng. Chem. Res. 2007, 46, 997–1012. [Google Scholar]
- Reich, S.; Thomsen, C.; Maultzsch, J. Carbon Nanotubes: Basic Concepts and Physical Properties; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Hatton, R.A.; Miller, A.J.; Silva, S.R.P. Carbon nanotubes: A multi-functional material for organic optoelectronics. J. Mater. Chem. 2008, 18, 1183–1192. [Google Scholar]
- Ren, S.Q.; Bernardi, M.; Lunt, R.; Bulovic, V.; Grossman, J.C.; Gradecak, S. Towards Efficient Semiconducting Carbon Nanotubes/P3HT Solar Cells, Insights into the Nanoscale Chemistry and Physics. Nano Lett. 2011, 11, 5316–5321. [Google Scholar]
- Bell, J.M.; Goh, R.G.S.; Waclawik, E.R.; Giulianini, M.; Motta, N. Polymer-carbon nanotube composites: Basic science and applications. Mater. Forum 2008, 32, 144–152. [Google Scholar]
- Capasso, A.; Salamandra, L.; di Carlo, A.; Bell, J.M.; Motta, N. Low-temperature synthesis of carbon nanotubes on indium tin oxide electrodes for organic solar cells. Beilstein J. Nanotechnol. 2012, 3, 524–532. [Google Scholar] [Green Version]
- Schuettfort, T.; Snaith, H.J.; Nish, A.; Nicholas, R.J. Synthesis and spectroscopic characterization of solution processable highly ordered polythiophene-carbon nanotube nanohybrid structures. Nanotechnology 2010, 21, 025201–025206. [Google Scholar]
- Kymakis, E.; Amaratunga, G.A.J. Single-wall carbon nanotube/conjugated polymer photovoltaic devices. Appl. Phys. Lett. 2002, 80, 112–114. [Google Scholar]
- Miller, A.J.; Hatton, R.A.; Silva, S.R.P. Water-soluble multiwall-carbon-nanotube-polythiophene composite for bilayer photovoltaics. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef] [Green Version]
- Barnes, T.M.; Bergeson, J.D.; Tenent, R.C.; Larsen, B.A.; Teeter, G.; Jones, K.M.; Blackburn, J.L.; van de Lagemaat, J. Carbon nanotube network electrodes enabling efficient organic solar cells without a hole transport layer. Appl. Phys. Lett. 2010, 96, 243309. [Google Scholar]
- Patyk, R.L.; Lomba, B.S.; Nogueira, A.F.; Furtado, C.A.; Santos, A.P.; Mello, R.M.Q.; Micaroni, L.; Hummelgen, I.A. Carbon nanotube-polybithiophene photovolyaic devices with high open-circuit voltage. Phys. Status Solidi 2007, 1, R43–R45. [Google Scholar]
- Feng, Y.; Ju, X.; Feng, W.; Zhang, H.; Cheng, Y.; Liu, J.; Fujii, A.; Ozaki, M.; Yoshino, K. Organic solar cells using few-walled carbon nanotubes electrode controlled by the balance between sheet resistance and the transparency. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Kim, S.; Yim, J.; Wang, X.; Bradley, D.D.C.; Lee, S.; deMello, J.C. Spin- and spray-deposited single-walled carbon-nanotube electrodes for organic solar cells. Adv. Funct. Mater. 2010, 20, 2310–2316. [Google Scholar]
- Rowell, M.W.; Topinka, M.A.; McGehee, M.D.; Prall, H.-J.; Dennler, G.; Sariciftci, N.S.; Hu, L.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Unalan, H.E.; Hiralal, P.; Kuo, D.; Parekh, B.; Amaratunga, G.; Chhowalla, M. Flexible organic photovoltaics from zinc oxide nanowires grown on transparent and conducting single walled carbon nanotube thin films. J. Mater. Chem. 2008, 18, 5909–5912. [Google Scholar]
- Miller, A.J.; Hatton, R.A.; Chen, G.Y.; Silva, S.R.P. Carbon nanotubes grown on In2O3: Sn glass as large area electrodes for organic photovoltaics. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef] [Green Version]
- Berson, S.; de Bettignies, R.; Bailly, S.; Guillerez, S.; Jousselme, B. Elaboration of p3ht/cnt/pcbm composites for organic photovoltaic cells. Adv. Funct. Mater. 2007, 17, 3363–3370. [Google Scholar]
- Li, C.; Mitra, S. Processing of fullerene-single wall carbon nanotube complex for bulk heterojunction photovoltaic cells. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Lanzi, M.; Paganin, L.; Caretti, D. New photoactive oligo- and poly-alkylthiophenes. Polymer 2008, 49, 4942–4948. [Google Scholar]
- Kymakis, E.; Kornilios, N.; Koudoumas, E. Carbon nanotube doping of P3HT: PCBM photovoltaic devices. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Picard, L.; Lincker, F.; Kervella, Y.; Zagorska, M.; DeBettignies, R.; Peigney, A.; Flahaut, E.; Louarn, G.; Lefrant, S.; Demadrille, R.; et al. Composites of double-walled carbon nanotubes with bis-quaterthiophene-fluorenone conjugated oligomer: Spectroelectrochemical and photovoltaic properties. J. Phys. Chem. C 2009, 113, 17347–17354. [Google Scholar]
- Hatton, R.A.; Blanchard, N.P.; Tan, L.W.; Latini, G.; Cacialli, F.; Silva, S.R.P. Oxidised carbon nanotubes as solution processable, high work function hole-extraction layers for organic solar cells. Org. Electron. 2009, 10, 388–395. [Google Scholar]
- Adikaari, A.A.D.T.; Dissanayake, D.M.N.M.; Silva, S.R.P. Organic-inorganic solar cells: Recent developments and outlook. Sel. Top. Quantum Electron. IEEE J. 2010, 16, 1595–1606. [Google Scholar]
- Capasso, A.; Salamandra, L.; Chou, A.; di Carlo, A.; Motta, N. Multi-wall carbon nanotube coating of fluorine-doped tin oxide as an electrode surface modifier for polymer solar cells. Sol. Energy Mater. Sol. Cells 2014, 122, 297–302. [Google Scholar] [Green Version]
- Yan, J.; Ni, T.; Zou, F.; Zhang, L.; Yang, D.; Yang, S.; Zou, B. Towards optimization of functionalized single-walled carbon nanotubes adhering with poly(3-hexylthiophene) for highly efficient polymer solar cells. Diam. Relat. Mater. 2014, 41, 79–83. [Google Scholar]
- Lee, I.; Lee, S.; Kim, H.; Lee, H.; Kim, Y. Polymer solar cells with polymer/carbon nanotube composite hole-collecting buffer layers. Open Phys. Chem. J. 2010, 4, 1–3. [Google Scholar]
- Lee, R.-H.; Lee, L.-Y.; Huang, J.-L.; Huang, C.-C.; Hwang, J.-C. Conjugated polymer-functionalized carbon nanotubes enhance the photovoltaic properties of polymer solar cells. Colloid Polym. Sci. 2011, 289, 1633–1641. [Google Scholar]
- Sharma, A.; Andersson, G.; Lewis, D.A. Role of humidity on indium and tin migration in organic photovoltaic devices. Phys. Chem. Chem. Phys. 2011, 13, 4381–4387. [Google Scholar]
- Choi, G.S.; Cho, Y.S.; Son, K.H.; Kim, D.J. Mass production of carbon nanotubes using spin-coating of nanoparticles. Microelectron. Eng. 2003, 66, 77–82. [Google Scholar]
- Dan, B.; Irvin, G.C.; Pasquali, M. Continuous and scalable fabrication of transparent conducting carbon nanotube films. ACS Nano 2009, 3, 835–843. [Google Scholar]
- De, S.; Lyons, P.E.; Sorel, S.; Doherty, E.M.; King, P.J.; Blau, W.J.; Nirmalraj, P.N.; Boland, J.J.; Scardaci, V.; Joimel, J.; et al. Transparent, flexible, and highly conductive thin films based on polymer-nanotube composites. ACS Nano 2009, 3, 714–720. [Google Scholar]
- Jo, J.W.; Jung, J.W.; Lee, J.U.; Jo, W.H. Fabrication of highly conductive and transparent thin films from single-walled carbon nanotubes using a new non-ionic surfactant via spin coating. ACS Nano 2010, 4, 5382–5388. [Google Scholar]
- Schindler, A.; Brill, J.; Fruehauf, N.; Novak, J.P.; Yaniv, Z. Solution-deposited carbon nanotube layers for flexible display applications. Phys. E Low Dimens. Syst. Nanostruct. 2007, 37, 119–123. [Google Scholar]
- Song, J.-W.; Kim, J.; Yoon, Y.-H.; Choi, B.-S.; Kim, J.-H.; Han, C.-S. Inkjet printing of single-walled carbon nanotubes and electrical characterization of the line pattern. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Zhang, D.; Ryu, K.; Liu, X.; Polikarpov, E.; Ly, J.; Tompson, M.E.; Zhou, C. Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes. Nano Lett. 2006, 6, 1880–1886. [Google Scholar]
- Zhou, Y.; Hu, L.; Grüner, G. A method of printing carbon nanotube thin films. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Ulbricht, R.; Jiang, X.; Lee, S.; Inoue, K.; Zhang, M.; Fang, S.; Baughman, R.; Zakhidov, A. Polymeric solar cells with oriented and strong transparent carbon nanotube anode. Phys. Status Solidi 2006, 243, 3528–3532. [Google Scholar]
- Gabor, N.M.; Zhong, Z.; Bosnick, K.; Park, J.; McEuen, P.L. Extremely Efficient Multiple Electron-Hole Pair Generation in Carbon Nanotube Photodiodes. Science 2009, 325, 1367–1371. [Google Scholar]
- Chaudhary, S.; Lu, H.; M1uller, A.M.; Bardeen, C.J.; Ozkan, M. Hierarchical placement and associated optoelectronic impact of carbon nanotubes in polymer-fullerene solar cells. Nano Lett. 2007, 7, 1973–1979. [Google Scholar]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar]
- Kymakis, E.; Klapsis, G.; Koudoumas, E.; Stratakis, E.; Kornilios, N.; Vidakis, N.; Franghiadakis, Y. Carbon nanotube/PEDOT: PSS electrodes for organic photovoltaics. Eur. Phys. J. Appl. Phys. 2006, 36, 257–259. [Google Scholar]
- Ulbricht, R.; Lee, S.B.; Jiang, X.; Inoue, K.; Zhang, M.; Fang, S.; Baughman, R.H.; Zakhidov, A.A. Transparent carbon nanotube sheets as 3-d charge collectors in organic solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 416–419. [Google Scholar]
- Van De Lagemaat, J.; Barnes, T.M.; Rumbles, G.; Shaheen, S.E.; Coutts, T.J.; Weeks, C.; Levitsky, I.; Peltola, J.; Glatkowski, P. Organic solar cells with carbon nanotubes replacing In2O3:Sn as the transparent electrode. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Ago, H.; Petritsch, K.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Composites of carbon nanotubes and conjugated polymers for photovoltaic devices. Adv. Mater. 1999, 11, 1281–1285. [Google Scholar]
- Pasquier, A.D.; Unalan, H.E.; Kanwal, A.; Miller, S.; Chhowalla, M. Conducting and transparent single-wall carbon nanotube electrodes for polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 87, 1–3. [Google Scholar]
- Kymakis, E.; Stratakis, E.; Koudoumas, E. Integration of carbon nanotubes as hole transport electrode in polymer/fullerene bulk heterojunction solar cells. Thin Solid Films 2007, 515, 8598–8600. [Google Scholar]
- Khatri, I.; Adhikari, S.; Aryal, H.R.; Soga, T.; Jimbo, T.; Umeno, M. Improving photovoltaic properties by incorporating both single walled carbon nanotubes and functionalized multiwalled carbon nanotubes. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Miller, A.J.; Hatton, R.A.; Silva, S.R.P. Interpenetrating multiwall carbon nanotube electrodes for organic solar cells. Appl. Phys. Lett. 2006, 89, 133117–133119. [Google Scholar]
- Jackson, R.; Domercq, B.; Jain, R.; Kippelen, B.; Graham, S. Stability of doped transparent carbon nanotube electrodes. Adv. Funct. Mater. 2008, 18, 2548–2554. [Google Scholar]
- Parekh, B.B.; Fanchini, G.; Eda, G.; Chhowalla, M. Improved conductivity of transparent single-wall carbon nanotube thin films via stable post deposition functionalization. Appl. Phys. Lett. 2007, 90, 121913–121913. [Google Scholar]
- Cho, D.-Y.; Eun, K.; Choa, S.-H.; Kim, H.-K. Highly flexible and stretchable carbon nanotube network electrodes prepared by simple brush painting for cost-effective flexible organic solar cells. Carbon 2014, 66, 530–538. [Google Scholar]
- Hatton, R.A.; Blanchard, N.P.; Stolojan, V.; Miller, A.J.; Silva, S.R.P. Nanostructured copper phthalocyanine-sensitized multiwall carbon nanotube films. Langmuir 2007, 23, 6424–6430. [Google Scholar]
- Tanaka, S.; Mielczarek, K.; Ovalle-Robles, R.; Wang, B.; Hsu, D.; Zakhidov, A.A. Monolithic parallel tandem organic photovoltaic cell with transparent carbon nanotube interlayer. Appl. Phys. Lett. 2009, 94, 113503–113506. [Google Scholar]
- Pradhan, B.; Batabyal, S.K.; Pal, A.J. Functionalized carbon nanotubes in donor/acceptor-type photovoltaic devices. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kymakis, E.; Amaratunga, G.A.J. Photovoltaic properties of dye functionalized single-wall carbon nanotube/conjugated polymer devices. Chem. Mater. 2004, 16, 4819–4823. [Google Scholar]
- Rahman, G.M.A.; Guldi, D.M.; Cagnoli, R.; Mucci, A.; Schenetti, L.; Vaccari, L.; Prato, M. Combining single wall carbon nanotubes and photoactive polymers for photoconversion. J. Am. Chem. Soc. 2005, 127, 10051–10057. [Google Scholar]
- Gregg, B.A. Excitonic solar cells. J. Phys. Chem. B 2003, 107, 4688–4698. [Google Scholar]
- Kymakis, E.; Alexandrou, I.; Amaratunga, G.A.J. High open-circuit voltage photovoltaic devices from carbon-nanotube-polymer composites. J. Appl. Phys. 2003, 93, 1764–1768. [Google Scholar]
- Mao, J.; Liu, Q.; Lv, X.; Liu, Z.; Huang, Y.; Ma, Y.; Chen, Y.; Yin, S. A water-soluble hybrid material of single-walled carbon nanotubes with an amphiphilic poly(phenyleneethynylene): Preparation, characterization, and photovoltaic properties. J. Nanosci. Nanotechnol. 2007, 7, 2709–2718. [Google Scholar]
- Liu, Q.; Mao, J.; Liu, Z.; Zhang, N.; Wang, Y.; Yang, L.; Yin, S.; Chen, Y. A photovoltaic device based on a poly(phenyleneethynylene)/swnt composite active layer. Nanotechnology 2008, 19, 115601–115606. [Google Scholar]
- Yun, D.; Feng, W.; Wu, H.; Li, B.; Liu, X.; Yi, W.; Qiang, J.; Gao, S.; Yan, S. Controllable functionalization of single-wall carbon nanotubes by in situ polymerization method for organic photovoltaic devices. Synth. Met. 2008, 158, 977–983. [Google Scholar]
- Somani, S.P.; Somani, P.R.; Flahaut, E.; Kalita, G.; Umeno, M. Double-walled carbon nanotubes-incorporated donor–acceptor-type organic photovoltaic devices using poly(3-octylthiophene) and C60. Jpn. J. Appl. Phys. 2008, 47, 1219–1222. [Google Scholar]
- Reyes-Reyes, M.; López-Sandoval, R.; Liu, J.; Carroll, D.L. Bulk heterojunction organic photovoltaic based on polythiophene–polyelectrolyte carbon nanotube composites. Sol. Energy Mater. Sol. Cells 2007, 91, 1478–1482. [Google Scholar]
- Kymakis, E.; Amaratunga, G.A.J. Photovoltaic cells based on dye-sensitisation of single-wall carbon nanotubes in a polymer matrix. Sol. Energy Mater. Sol. Cells 2003, 80, 465–472. [Google Scholar]
- Chehata, N.; Ltaief, A.; Bkakri, R.; Bouazizi, A.; Beyou, E. Conducting polymer functionalized multi-walled carbon nanotubes nanocomposites: Optical properties and morphological characteristics. Mater. Lett. 2014, 121, 227–230. [Google Scholar]
- Chen, B.-C.; Cheng, Y.-S.; Gau, C.; Lee, Y.-C. Enhanced performance of polymer solar cells with imprinted nanostructures on the active layer. Thin Solid Films 2014, 564, 384–389. [Google Scholar]
- Arranz-Andrés, J.; Blau, W.J. Enhanced device performance using different carbon nanotube types in polymer photovoltaic devices. Carbon 2008, 46, 2067–2075. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alturaif, H.A.; ALOthman, Z.A.; Shapter, J.G.; Wabaidur, S.M. Use of Carbon Nanotubes (CNTs) with Polymers in Solar Cells. Molecules 2014, 19, 17329-17344. https://doi.org/10.3390/molecules191117329
Alturaif HA, ALOthman ZA, Shapter JG, Wabaidur SM. Use of Carbon Nanotubes (CNTs) with Polymers in Solar Cells. Molecules. 2014; 19(11):17329-17344. https://doi.org/10.3390/molecules191117329
Chicago/Turabian StyleAlturaif, Huda A., Zeid A. ALOthman, Joseph G. Shapter, and Saikh M. Wabaidur. 2014. "Use of Carbon Nanotubes (CNTs) with Polymers in Solar Cells" Molecules 19, no. 11: 17329-17344. https://doi.org/10.3390/molecules191117329




