Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binding of Resveratrol to Albumin Followed by Resveratrol and by Protein Tryptophanyl Fluorescences
| Albumin Form | Concentration (µM) | Scatchard Analysis | Stern Volmer Analysis | ||||
|---|---|---|---|---|---|---|---|
| Type 1 Sites Number (n) | KD Type 1 (µM) | Type 2 Sites Number (n) | KD Type 2 (µM) | KD Type 2 (µM) | FluorophoresNumber | ||
| Free FA | 0.1 | 0.8 | 0.02 | 1 | 0.05 | 0.06 | 2 |
| Free FA | 1 | 0.8 | 0.6 | 1 | 1.44 | 1.76 | 2 |
| Free FA | 1.5 | 0.6 | 4.8 | 1.2 | 7.69 | 12.71 | 2 |
| Free FA | 3 | 1.1 | 4.8 | - | - | 16.23 | 2 |
| Fraction V | 1 | 1.1 | 1.16 | 2.5 | 16.7 | 21.6 | 2 |
2.2. Determination of Binding Site Number and Dissociation Constants of Resveratrol to Albumin
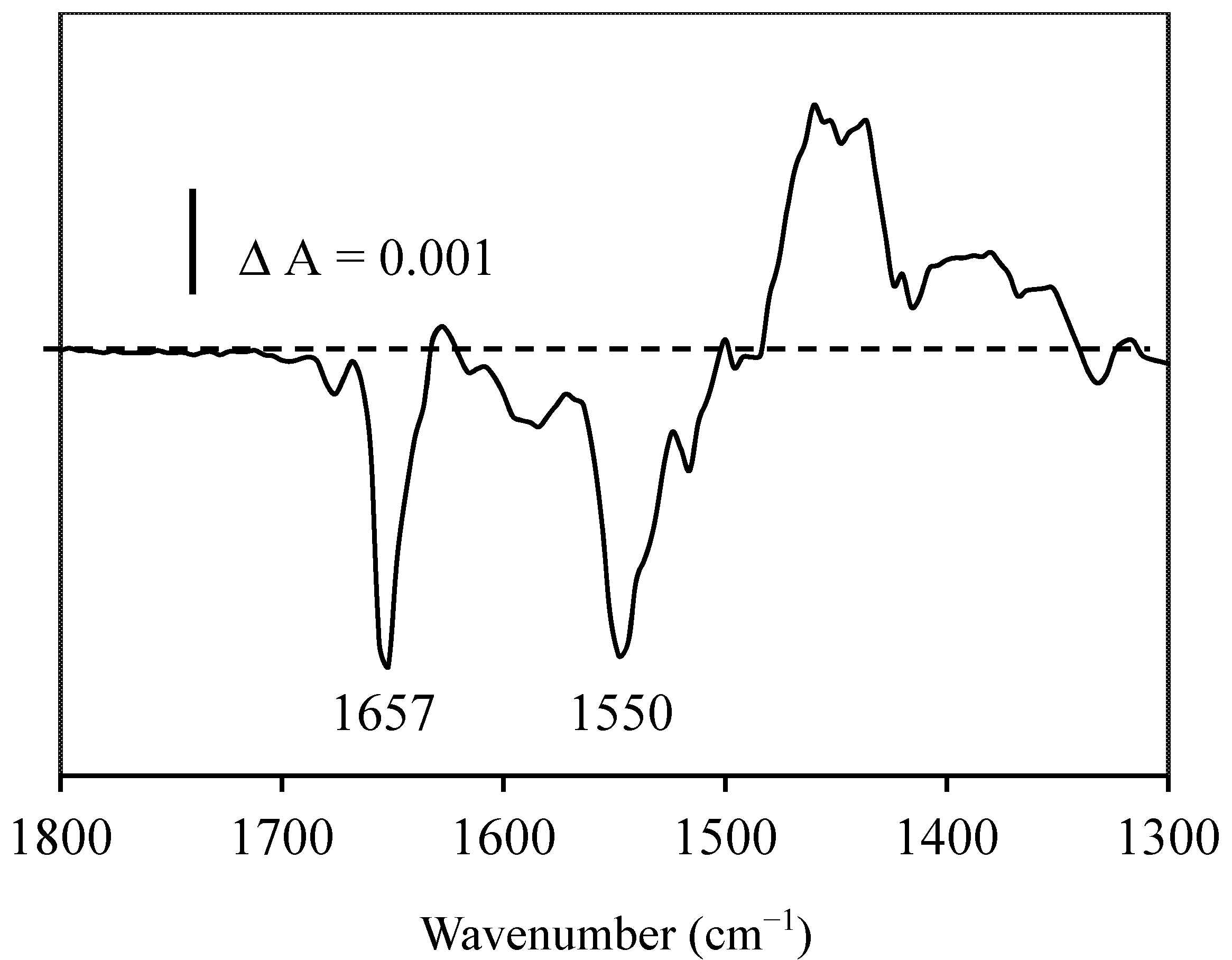
2.3. Resveratrol-Albumin Interactions Observed by Infrared Spectroscopy
2.4. Discussion
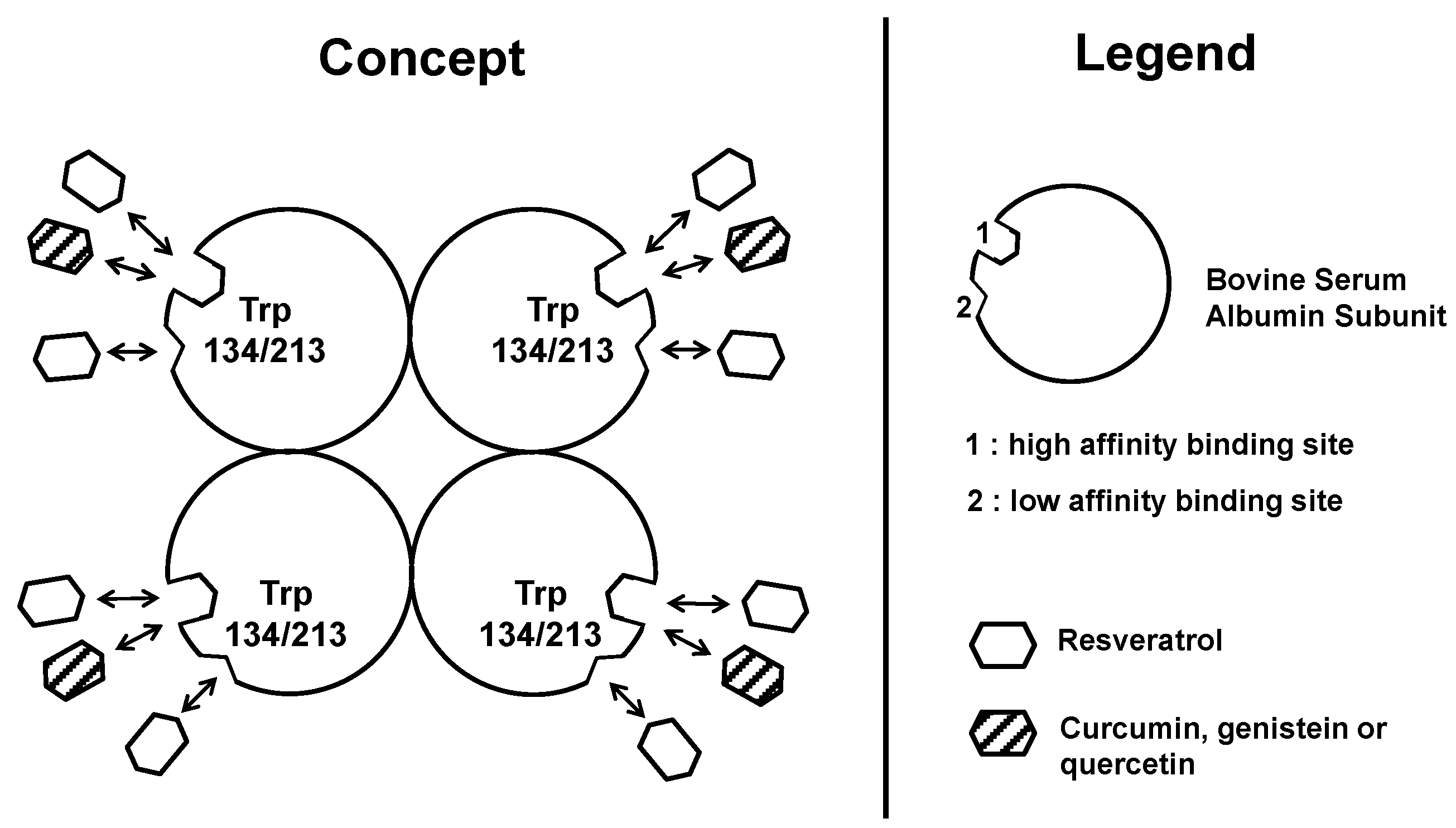
2.4.1. Resveratrol Interactions with BSA or HAS
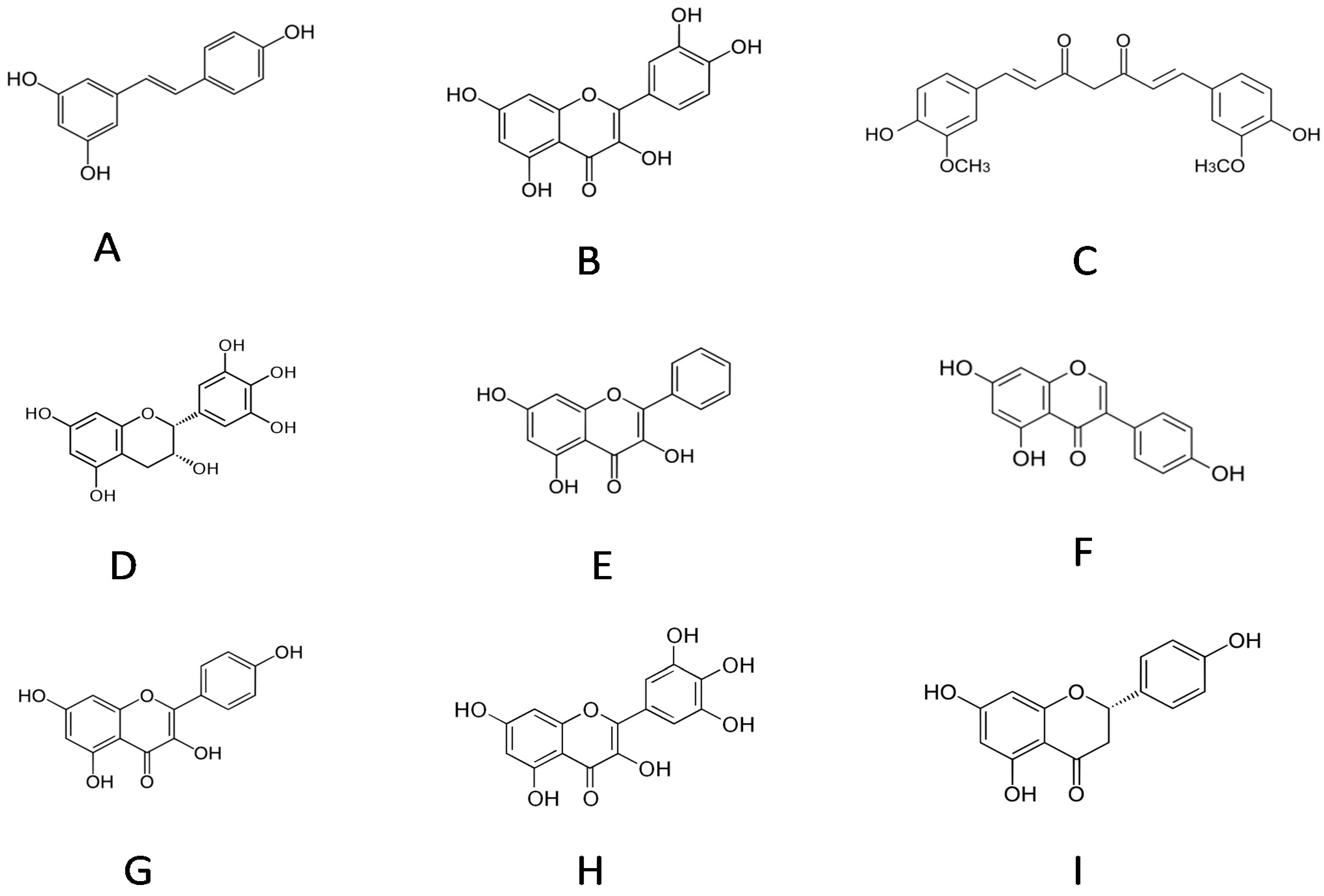
2.4.2. Comparisons with Other Dietary Polyphenol, such as Quercetin
2.4.3. Comparisons with Flavonoids, such as Curcumin, Epigallocatechin, Galangin, Genistein, Kaempferol, Myricetin and Naringenin
| Polyphenol | Type 1 Sites Number (n) | KD Type 1 (µM) | Type 2 Sites Number (n) | KD Type 2 (µM) | Ref. |
|---|---|---|---|---|---|
| Resveratrol | 1 | 4 ± 0.4 | 2 | 20 ± 2 | This paper (Menzel, 2014) |
| Resveratrol | 1.3 | 253 ± 50 | [27] | ||
| Resveratrol | 25 | [26] | |||
| Curcumin | 1 | 333 ± 80 | [27] | ||
| Genistein | 1.3 | 126 ± 30 | [27] | ||
| Quercetin | 0.36 | [40] | |||
| Quercetin | Yes | 19 | Yes | ? | [32,33] |
| Myricetin | 0.049 | [40] |
3. Materials and Methods
3.1. Chemicals
3.2. Fluorescence
3.3. Infrared Spectra
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomera, J.F. Current knowledge of the health benefits and disadvantages of wine consumption. Trends Food Sci. Technol. 1999, 10, 129–138. [Google Scholar]
- Surh, Y.J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999, 428, 305–327. [Google Scholar]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar]
- Latruffe, N.; Delmas, D.; Passilly-Degrace, P.; Jannin, B.; Cherkaoui Malki, M.; Berlot, J.P. Molecular analysis on the chemopreventive properties of resveratrol, a plant polyphenol microcomponent. Int. J. Mol. Med. 2002, 10, 755–760. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.R.; Zipkin, E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar]
- Renaud, S.C.; Guéguen, R.; Schenker, J.; d’Houtaud, A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology 1998, 9, 184–188. [Google Scholar]
- Delmas, D.; Jannin, B.; Cherkaoui Malki, M.; Latruffe, N. Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol. Rep. 2000, 7, 847–852. [Google Scholar]
- Delmas, D.; Passilly-Degrace, P.; Jannin, B.; Cherkaoui Malki, M.; Latruffe, N. Resveratrol, a chemopreventive agent, disrupts the cell cycle control of human SW480 colorectal tumor cells. Int. J. Mol. Med. 2002, 10, 193–139. [Google Scholar]
- Delmas, D.; Rebe, C.; Lacour, S.; Filomenko, R.; Athias, A.; Gambert, P.; Cherkaoui-Malki, M.; Jannin, B.; Dubrez-Daloz, L.; Latruffe, N.; et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death inducing signaling complex in colon cancer cells. J. Biol. Chem. 2003, 278, 41482–41490. [Google Scholar]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 57, 1347–1355. [Google Scholar]
- Khan, M.A.; Muzammil, S.; Musarrat, J. Differential binding of tetracyclines with serum albuminand induced structural alterations in drug-bound protein. Int. J. Biol. Macromol. 2002, 30, 243–249. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1983. [Google Scholar]
- Lançon, A.; Delmas, D.; Osman, H.; Thénot, J.P.; Jannin, B.; Latruffe, N. Study of resveratrol uptake and efflux in hepatic cells by fluorescence microscopy and radiolabeling. Biochem. Biophys. Res. Commun. 2004, 316, 1132–1137. [Google Scholar]
- Jannin, B.; Menzel, M.; Berlot, J.P.; Delmas, D.; Lançon, A.; Latruffe, N. Interactions of resveratrol, a chemopreventive agent, with serum albumin. Biochem. Pharmacol. 2004, 68, 1113–1118. [Google Scholar]
- Scatchard, G. The attraction of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949, 51, 660–672. [Google Scholar]
- Mantele, W. Reaction-induced infrared difference spectroscopy for the study of protein function and reaction mechanisms. Trends Biochem. Sci. 1993, 18, 197–202. [Google Scholar]
- Haris, P.I.; Chapman, D. The conformational analysis of peptides using Fourier transform IRspectroscopy. Biopolymers 1995, 37, 251–263. [Google Scholar]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar]
- Curry, S.; Brick, P.; Franks, N.P. Fatty acid binding to human serum albumin: New insights from crystallographic studies. Biochim. Biophys. Acta 1999, 1441, 131–140. [Google Scholar]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar]
- Ozer, I.; Tacal, O. Method dependence of apparent stoichiometry in the binding of salicylate ion to human serum albumin: A comparison between equilibrium dialysis and fluorescence titration. Anal. Biochem. 2001, 294, 1–6. [Google Scholar]
- Sulkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar]
- Urpí-Sardà, M.; Jáuregui, O.; Lamuela-Raventós, R.M.; Jaeger, W.; Miksits, M.; Covas, M.I.; Andres-Lacueva, C. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal. Chem. 2005, 77, 3149–3155. [Google Scholar]
- Richard, T.; Vitrac, X.; Merillon, J.M.; Monti, J.P. Role of peptide primary sequence in polyphenol-protein recognition: An example with neurotensin. Biochim. Biophys. Acta 2005, 1726, 238–243. [Google Scholar]
- Pantusa, M.; Sportelli, L.; Bartucci, R. Influence of stearic acids on resveratrol-HSA interaction. Eur. Biophys. J. 2012, 41, 969–977. [Google Scholar]
- N’Soukpoe-Kossi, C.N.; St-Louis, C.; Beauregard, M.; Subirade, M.; Carpentier, R.; Hotchandani, S.; Tajmir-Riahi, H.A. Resveratrol binding to human serum albumin. J. Biomol. Struct. Dyn. 2006, 24, 277–283. [Google Scholar]
- Bourassa, P.; Kanakis, C.D.; Tarantilis, P.; Pollissiou, M.G.; Tajmir-Riahi, H.A. Resveratrol, genistein, and curcumin bind bovine serum albumin. J. Phys. Chem. B 2010, 114, 3348–3354. [Google Scholar]
- Lu, Z.; Zhang, Y.; Liu, H.; Yuan, J.; Zheng, Z.; Zou, G. Transport of a cancer chemopreventive polyphenol, resveratrol: Interaction with serum albumin and hemoglobin. J. Fluoresc. 2007, 17, 580–587. [Google Scholar]
- Levine, R.L. Fluorescence-quenching studies of the binding of bilirubin to albumin. Clin. Chem. 1977, 23, 2292–2301. [Google Scholar]
- Pantusa, M.; Bartucci, R.; Rizzuti, B. Stability of trans-Resveratrol Associated with Transport Protein. J. Agric. Food Chem. 2014, 62, 4384–4391. [Google Scholar]
- Dawra, R.K.; Makkar, H.P.; Singh, B. Protein-binding capacity of microquantities of tannins. Anal. Biochem. 1988, 170, 50–53. [Google Scholar]
- Sengupta, B.; Sengupta, P.K. The interaction of quercetin with human serum albumin: A fluorescence spectroscopic study. Biochem. Biophys. Res. Commun. 2002, 299, 400–403. [Google Scholar]
- Sengupta, B.; Sengupta, P.K. Binding of quercetin with human serum albumin: A critical spectroscopic study. Biopolymers 2003, 72, 427–434. [Google Scholar]
- Kitson, T.M. Spectrophotometric and kinetic studies on the binding of the bioflavonoid quercetin to bovine serum albumin. Biosci. Biotechnol. Biochem. 2004, 68, 2165–2170. [Google Scholar]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar]
- Kaldas, M.I.; Walle, U.K.; van der Woude, H.; McMillan, J.M.; Walle, T. Covalent binding of the flavonoid quercetin to human serum albumin. J. Agric. Food Chem. 2005, 53, 4194–4197. [Google Scholar]
- Dufour, C.; Dangles, O. Flavonoid-serum albumin complexation: Determination of binding constants and binding sites by fluorescence spectroscopy. Biochim. Biophys. Acta 2005, 1721, 164–173. [Google Scholar]
- Rolinski, O.J.; Martin, A.; Birch, D.J. Human serum albumin and quercetin interactions monitored by time-resolved fluorescence: Evidence for enhanced discrete rotamer conformations. J. Biomed. Opt. 2007, 12, 034013-1–034013-7. [Google Scholar]
- Martini, S.; Bonechi, C.; Rossi, C. Interaction of quercetin and its conjugate quercetin 3-O-beta-d-glucopyranoside with albumin as determined by NMR relaxation data. J. Nat. Prod. 2008, 71, 175–178. [Google Scholar]
- Xiao, J.; Suzuki, M.; Jiang, X.; Chen, X.; Yamamoto, K.; Ren, F.; Xu, M. Influence of B-ring hydroxylation on interactions of flavonols with bovine serum albumin. J. Agric. Food Chem. 2008, 56, 2350–2356. [Google Scholar]
- Skrt, M.; Benedik, E.; Podlipnik, C.; Ulrih, N.P. Interactions of different polyphenols with bovine serum albumin using fluorescence quenching and molecular docking. Food Chem. 2012, 135, 2418–2424. [Google Scholar]
- Kim, H.G.; Lee, J.H.; Lee, S.J.; Oh, J.H.; Shin, E.; Jang, Y.P.; Lee, Y.J. The increased cellular uptake and biliary excretion of curcumin by quercetin: A possible role of albumin binding interaction. Drug Metab. Dispos. 2012, 40, 1452–1455. [Google Scholar]
- Azzi, A. The Use of Fluorescent Probes for the Study of Membranes. Methods Enzymol. 1974, 32B, 234–246. [Google Scholar]
- Dahlquist, F.W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978, 48, 270–299. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latruffe, N.; Menzel, M.; Delmas, D.; Buchet, R.; Lançon, A. Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents. Molecules 2014, 19, 17066-17077. https://doi.org/10.3390/molecules191117066
Latruffe N, Menzel M, Delmas D, Buchet R, Lançon A. Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents. Molecules. 2014; 19(11):17066-17077. https://doi.org/10.3390/molecules191117066
Chicago/Turabian StyleLatruffe, Norbert, Matthias Menzel, Dominique Delmas, René Buchet, and Allan Lançon. 2014. "Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents" Molecules 19, no. 11: 17066-17077. https://doi.org/10.3390/molecules191117066





