Accumulation of Kaempferitrin and Expression of Phenyl-Propanoid Biosynthetic Genes in Kenaf (Hibiscus cannabinus)
Abstract
:1. Introduction

2. Results and Discussion
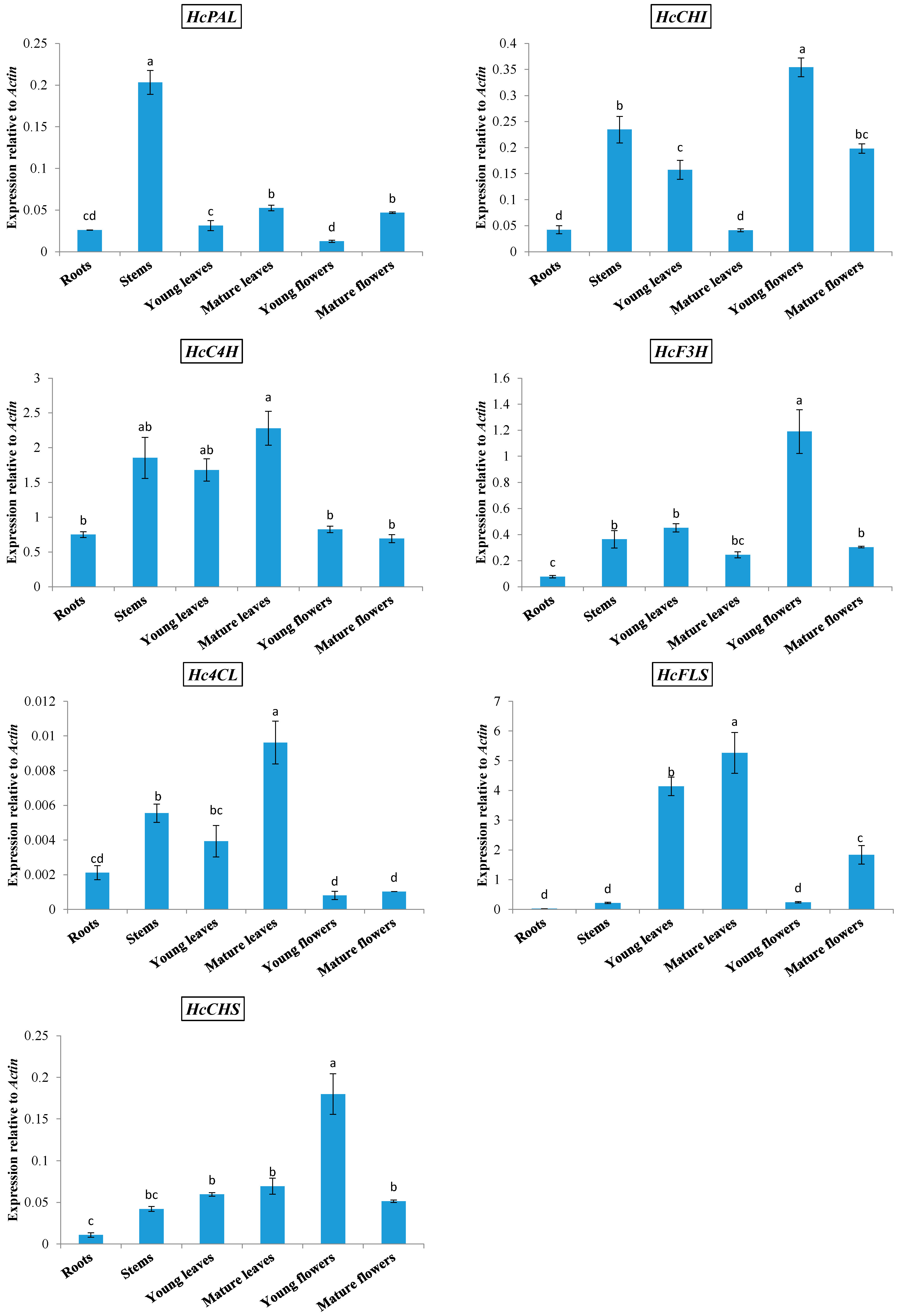
2.1. Expression of Phenylpropanoid Biosynthetic Genes in Kenaf
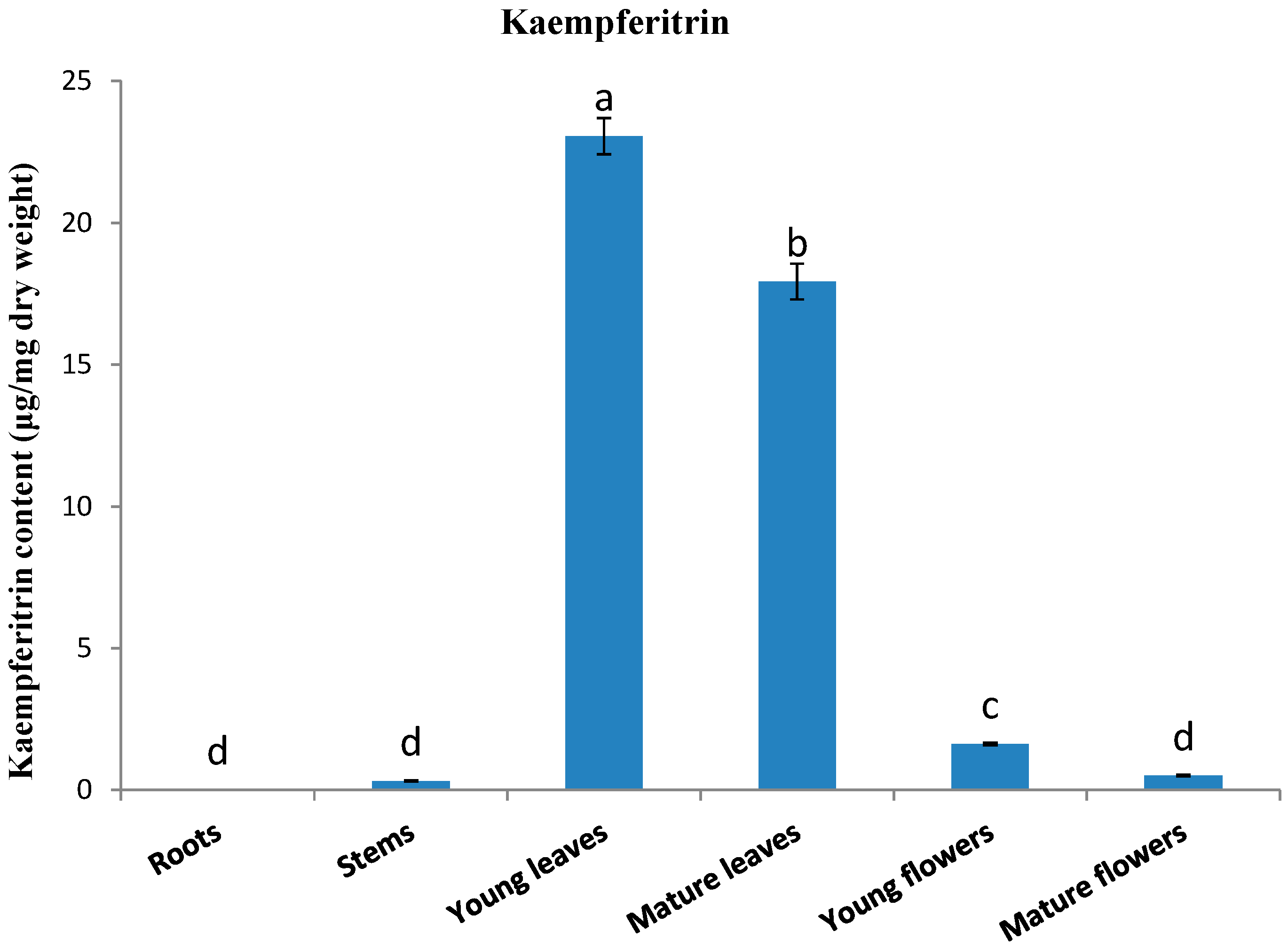
2.2. Kaempferitrin Content in Different Organs of Kenaf
3. Experimental Section
3.1. Plant Materials
3.2. Chemicals and Solvents
3.3. RNA Extraction and cDNA Synthesis
3.4. QRT-PCR Analysis
| Primer | Sequence (5' to 3') | Amplicon (Base Pairs) |
|---|---|---|
| HcPAL F | ACAGGAAGCCTTTCGTCTAGC | 218 |
| HcPAL R | TATGTGTCAAGTGGTCGGTGA | |
| HcC4H F | GTAGATTGGCACAGAGCTTCG | 198 |
| HcC4H R | CGATGGCACATTTAAGAGCAT | |
| Hc4CL F | TGAATTCATCTTTCGGTCCAG | 221 |
| Hc4CL R | GCATGATGACATCTCCCTGTT | |
| HcCHS F | GGGCTTACATTTCACCTCCTC | 225 |
| HcCHS R | GTTCCCGTATTCCGAAAGAAC | |
| HcCHI F | GTAAGAAAGCCGAGGAGTTGG | 190 |
| HcCHI R | GCCTTTTCTTCTGCATCAGTG | |
| HcF3H F | TGTGTGGACATGGATCAGAAA | 214 |
| HcF3H R | GATCTCCAAGGTTGACCACAA | |
| HcFLS F | AACCAGATGTGCTGAAACAGG | 239 |
| HcFLS R | CTGGTCGCCAATGTGAATAAT | |
| HcActin F | GTCTAGACCTTGCTGGTCGTG | 210 |
| HcActin R | CTTGTCCATCAGGCAATTCAT |
3.5. Extraction and Quantification of Kaempferitrin
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Kim, J.K.; Park, S.Y.; Zhao, S.; Kim, Y.B.; Lee, S.; Park, S.U. Comparative analysis of flavonoids and polar metabolite profiling of tanno-original and tanno-high rutin buckwheat. J. Agric. Food Chem. 2014, 62, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Park, N.I.; Li, X.; Xu, H.; Kim, H.M.; Park, S.U. Molecular cloning and characterization of phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in the phenylpropanoid biosynthesis pathway in garlic (Allium sativum). J. Agric. Food Chem. 2010, 58, 10911–10917. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.J.; Park, S.U. Metabolic profiling of glucosinolates, anthocyanins, carotenoids, and other secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tuan, P.A.; Li, X.; Kim, Y.B.; Kim, H.; Park, C.G.; Yang, J.; Li, C.H.; Park, S.U. Identification of phenylpropanoid biosynthetic genes and phenylpropanoid accumulation by transcriptome analysis of Lycium chinense. BMC Genomics 2013, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.; Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [PubMed]
- Jeong, H.Y.; Sung, G.H.; Kim, J.H.; Yoon, J.Y.; Yang, Y.; Park, J.G.; Kim, S.H.; Yi, Y.S.; Yang, W.S.; Yoon, D.H.; et al. SYK and SRC are major pharmacological targets of a Cerbera manghas methanol extract with kaempferol-based anti-inflammatory activity. J. Ethnopharmacol. 2014, 151, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H. Mechanisms underlying apoptosis-inducing effects of kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H.I.; Choi, E.Y.; Helton, W.B.; Gairola, C.G.; Valentino, J. Impact of apigenin and kaempferol on human head and neck squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 214–220. [Google Scholar] [PubMed]
- Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215–21226. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-α-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar]
- Yin, R.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zazimalova, E.; Schaffner, A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014, 201, 466–475. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [PubMed]
- Onkokesung, N.; Reichelt, M.; van Doorn, A.; Schuurink, R.C.; van Loon, J.J.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 2014, 65, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.M. Fiber Crops; University Presses of Florida: Gainesville, FL, USA, 1975. [Google Scholar]
- Yang, Y.X.; Lu, H.L.; Zhan, S.S.; Deng, T.H.; Lin, Q.Q.; Wang, S.Z.; Yang, X.H.; Qiu, R.L. Using kenaf (Hibiscus cannabinus) to reclaim multi-metal contaminated acidic soil. Ying Yong Sheng Tai Xue Bao 2013, 24, 832–838. [Google Scholar] [PubMed]
- Lewy, M. Kenaf seed oil. J. Am. Oil Chem. Soc. 1947, 24, 3–5. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Hasanoglu, A.; Irmak, S.; Erbatur, O. Biofuel production by liquefaction of kenaf (Hibiscus cannabinus L.) biomass. Bioresour. Technol. 2014, 151, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, S.; Evlard, A.; Mhamdi, M.E.; Campanella, B.; Paul, R.; Bettaieb, T. Potential of kenaf (Hibiscus cannabinus L.) and corn (Zea mays L.) for phytoremediation of dredging sludge contaminated by trace metals. Biodegradation 2013, 24, 563–567. [Google Scholar] [PubMed]
- Yazan, L.S.; Foo, J.B.; Chan, K.W.; Tahir, P.M.; Ismail, M. Kenaf seed oil from supercritical carbon dioxide fluid extraction shows cytotoxic effects towards various cancer cell lines. Afr. J. Biotechnol. 2011, 10, 5381–5388. [Google Scholar]
- Yusri, N.M.; Chan, K.W.; Iqbal, S.; Ismail, M. Phenolic content and antioxidant activity of Hibiscus cannabinus L. seed extracts after sequential solvent extraction. Molecules 2012, 17, 12612–12621. [Google Scholar] [PubMed]
- Agbor, G.A.; Oben, J.E.; Blaise, N.; Takala, J.P.; Ngogang, J.Y. Hepato protective activity of Hibiscus cannabinus (Linn.) against carbon tetrachloride and paracetamol induced liver damange in rats. Pak. J. Biol. Sci. 2005, 8, 1397–1401. [Google Scholar] [CrossRef]
- Andersen, J.R.; Zein, I.; Wenzel, G.; Darnhofer, B.; Eder, J.; Ouzunova, M.; Lubberstedt, T. Characterization of phenylpropanoid pathway genes within European maize (Zea mays L.) inbreds. BMC Plant Biol. 2008, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- PetuniaSpitzer-Rimon, B.; Marhevka, E.; Barkai, O.; Marton, I.; Edelbaum, O.; Masci, T.; Prathapani, N.K.; Shklarman, E.; Ovadis, M.; Vainstein, A. EOBII, a gene encoding a flower-specific regulator of phenylpropanoid volatiles' biosynthesis in petunia. Plant Cell 2010, 22, 1961–1976. [Google Scholar] [PubMed]
- Bharti, A.K.; Khurana, J.P. Mutants of Arabidopsis as tools to understand the regulation of phenylpropanoid pathway and UVB protection mechanisms. Photochem. Photobiol. 1997, 65, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-J.; Choi, B.S.; Bae, D.W.; Shin, S.C.; Park, S.U.; Lim, H.-S.; Kim, J.; Kim, J.B.; Cho, B.-K.; Bae, H. Differential expression of kenaf phenylalanine ammonia-lyase (PAL) ortholog during developmental stages and in response to abiotic stresses. Plant Omics 2012, 15, 392–399. [Google Scholar]
- Chowdhury, M.E. K.; Choi, B.; Cho, B.K.; Kim, J.B.; Park, S.U.; Natarajan, S.; Lim, H.S.; Bae, H. Regulation of 4CL, encoding 4-coumarate: Coenzyme A ligase, expression in kenaf under diverse stress conditions. Plant Omics 2013, 6, 254–262. [Google Scholar]
- Kim, J.; Choi, B.; Natarajan, S.; Bae, H. Expression analysis of kenaf cinnamate 4-hydroxylase (C4H) ortholog during developmental and stress responses. Plant Omics 2013, 6, 65–72. [Google Scholar]
- Rho, H.S.; Ahn, S.M.; Lee, B.C.; Kim, M.K.; Ghimeray, A.K.; Jin, C.W.; Cho, D.H. Changes in flavonoid content and tyrosinase inhibitory activity in kenaf leaf extract after far-infrared treatment. Bioorg. Med. Chem. Lett. 2010, 20, 7534–7536. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Shin, Y.; Tuan, P.A.; Li, X.; Park, Y.; Park, N.I.; Park, S.U. Molecular cloning and characterization of genes involved in rosmarinic acid biosynthesis from Prunella vulgaris. Biol. Pharm. Bull. 2014, 37, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, K.; Kim, Y.; Tuan, P.A.; Kim, H.H.; Cho, J.W.; Park, S.U. Cloning and characterization of a flavonol synthase gene from Scutellaria baicalensis. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.; Liu, Y. Determination of Kaempferitrin in Siraitia Grosvenori (Swingle) vine, leaf and extract by RP-HPLC. Mod. Chin. Med. 2010, 12, 25–31. [Google Scholar]
- Pinheiro, T.S.; Johansson, L.A.; Pizzolatti, M.G.; Biavatti, M.W. Comparative assessment of kaempferitrin from medicinal extracts of Bauhinia forficata link. J. Pharm. Biomed. Anal. 2006, 41, 431–436. [Google Scholar] [PubMed]
- Ghosh, R.; Choi, B.S.; Jeong, M.-J.; Bae, D.W.; Shin, S.C.; Park, S.U.; Lim, H.-S.; Kim, J.; Bae, H. Comparative transcriptional analysis of caffeoyl-coenzyme A 3-O-methyltransferase from Hibiscus cannabinus L., during developmental stages in various tissues and stress regulation. Plant Omics 2012, 5, 184–193. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Thwe, A.A.; Park, N.I.; Suzuki, T.; Kim, S.J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression during the development of Tartary Buckwheat Sprouts. J. Agric. Food Chem. 2012, 60, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: In general, samples of the compounds analyzed herein are unavailable from the authors due to their isolation on a small scale. They are readily analyzed using the procedures described.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Li, X.; Cho, D.H.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Accumulation of Kaempferitrin and Expression of Phenyl-Propanoid Biosynthetic Genes in Kenaf (Hibiscus cannabinus). Molecules 2014, 19, 16987-16997. https://doi.org/10.3390/molecules191016987
Zhao S, Li X, Cho DH, Arasu MV, Al-Dhabi NA, Park SU. Accumulation of Kaempferitrin and Expression of Phenyl-Propanoid Biosynthetic Genes in Kenaf (Hibiscus cannabinus). Molecules. 2014; 19(10):16987-16997. https://doi.org/10.3390/molecules191016987
Chicago/Turabian StyleZhao, Shicheng, Xiaohua Li, Dong Ha Cho, Mariadhas Valan Arasu, Naif Abdullah Al-Dhabi, and Sang Un Park. 2014. "Accumulation of Kaempferitrin and Expression of Phenyl-Propanoid Biosynthetic Genes in Kenaf (Hibiscus cannabinus)" Molecules 19, no. 10: 16987-16997. https://doi.org/10.3390/molecules191016987





