Effect of Tea Polyphenols on Lipid Peroxidation and Antioxidant Activity of Litchi (Litchi chinensis Sonn.) Fruit during Cold Storage
Abstract
:1. Introduction
2. Results
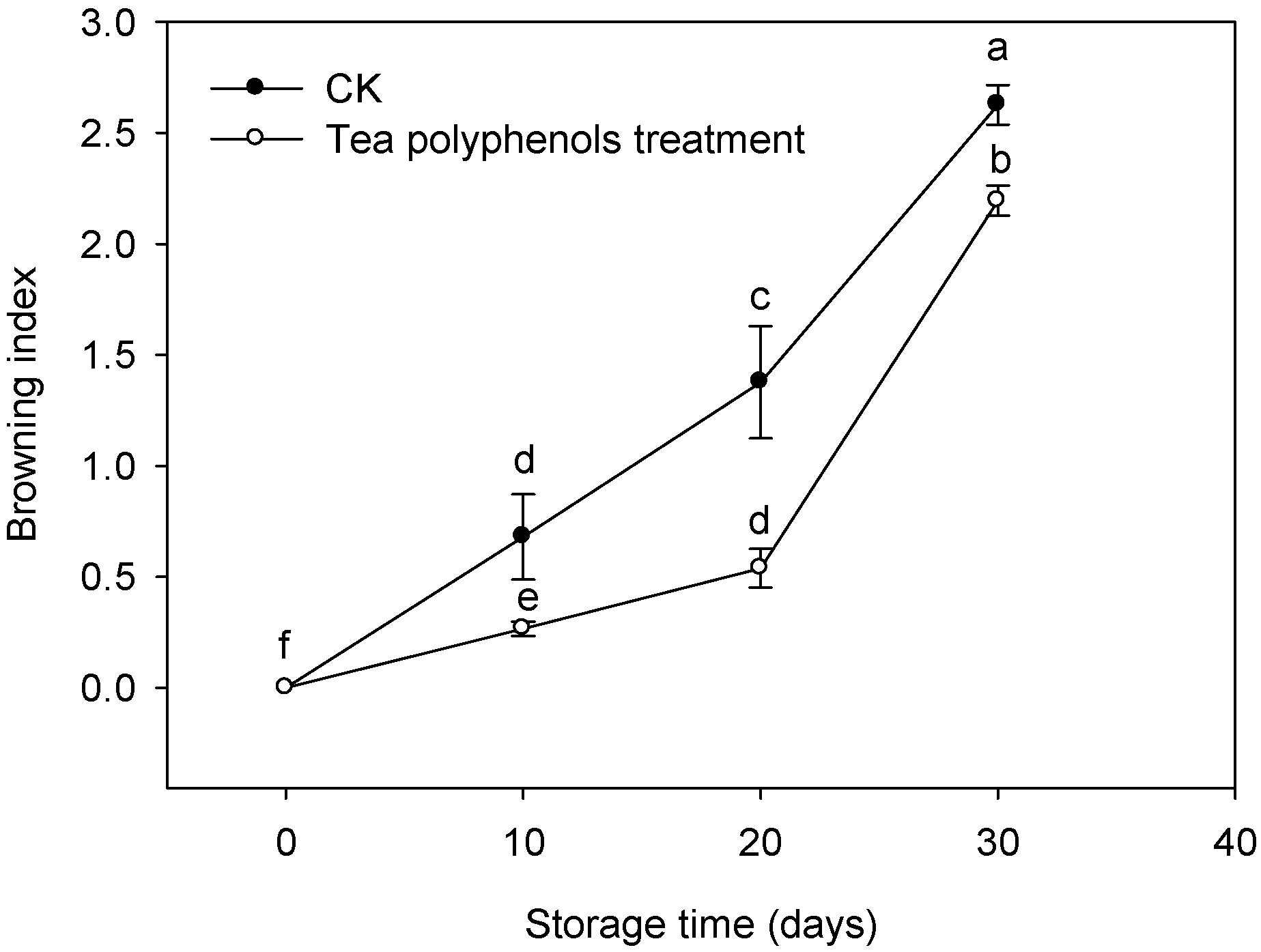
2.1. The Change in Browning Index
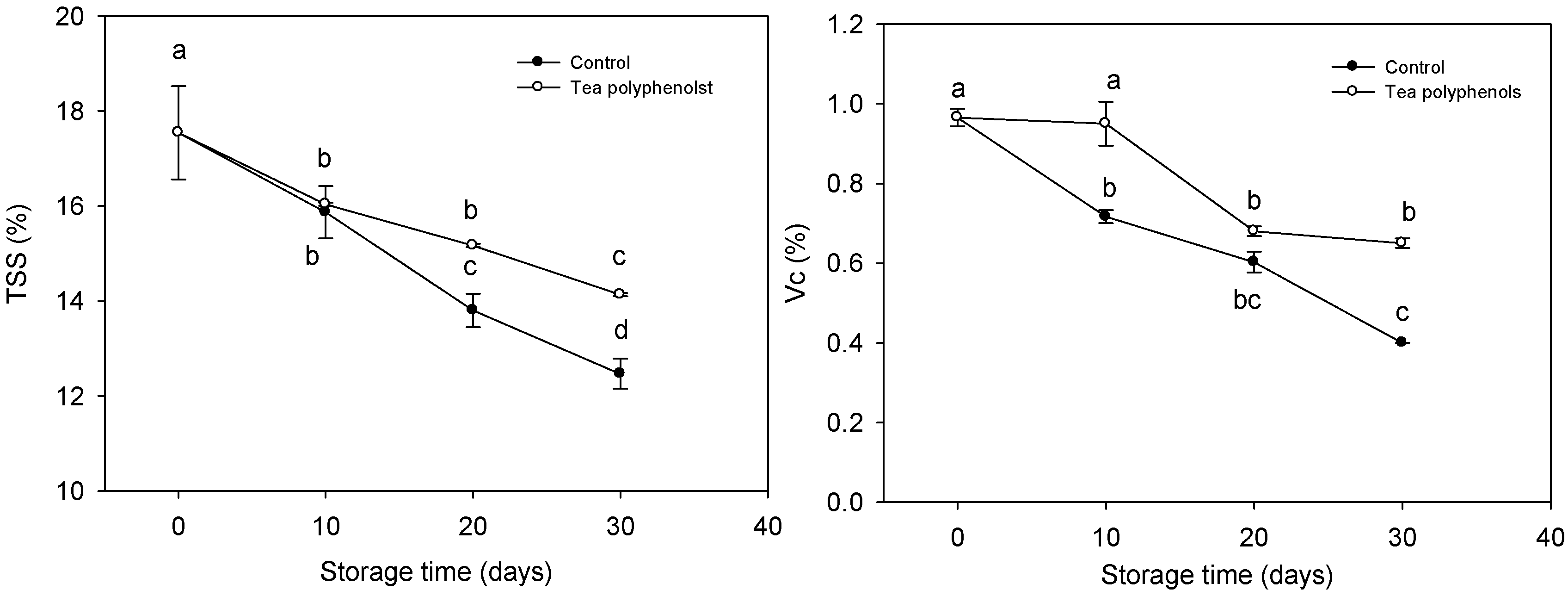
2.2. The Changes in Contents of Total Soluble Solids and Ascorbic Acid
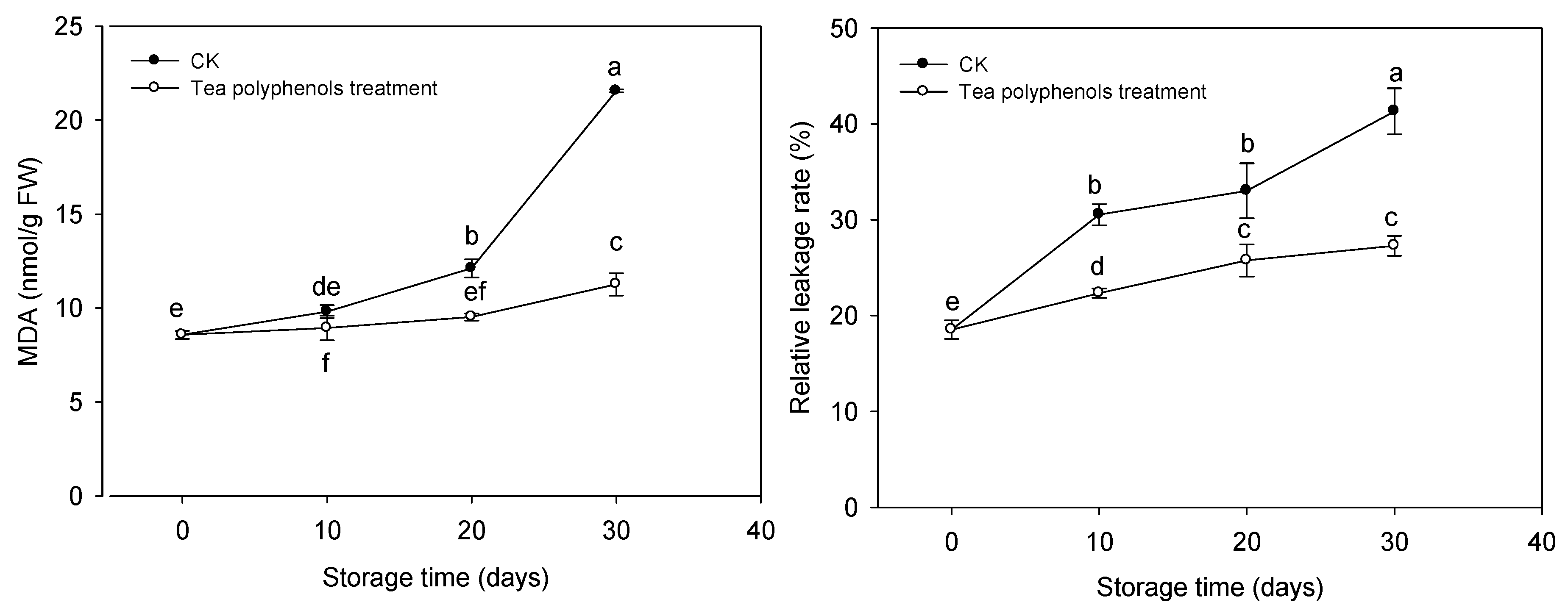
2.3. The Changes in Relative Electric Leakage Rate and Malondialdehyde Contents
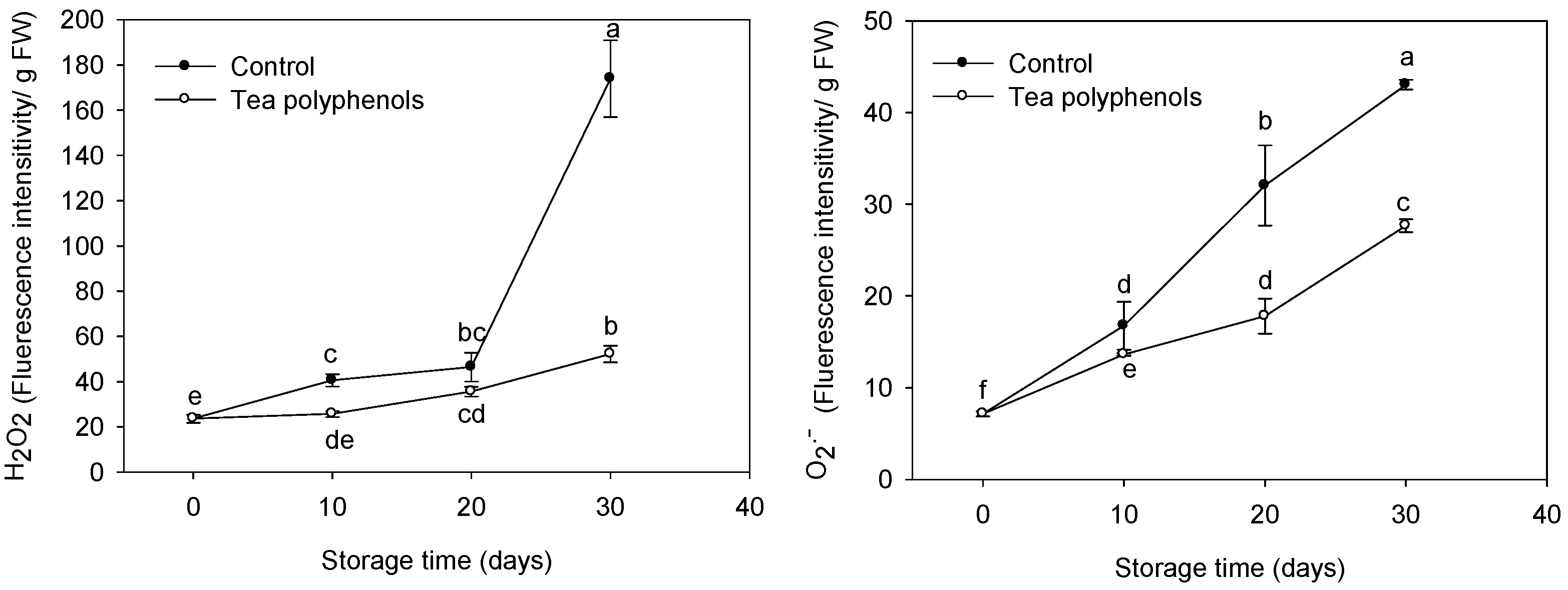
2.4. The Change in the Content of Reactive Oxygen Species
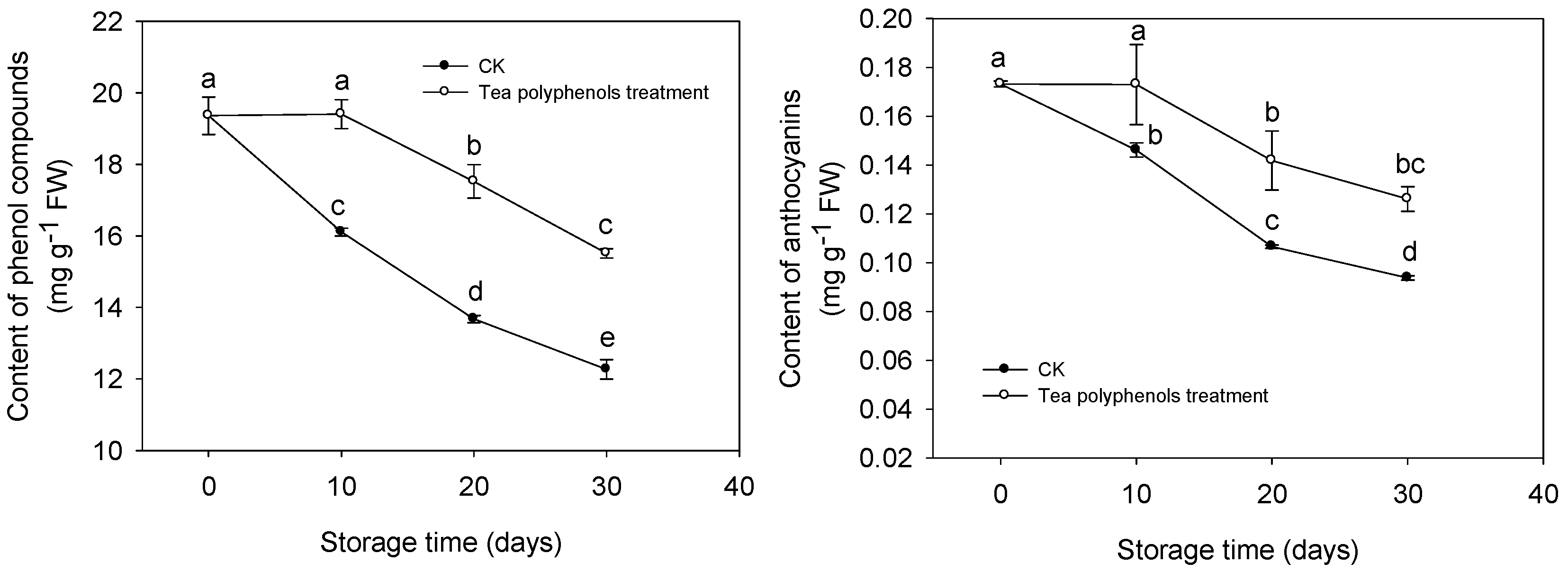
2.5. The Changes in the Contents of Total Phenols and Anthocyanins
2.6. The Change in the 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Activity

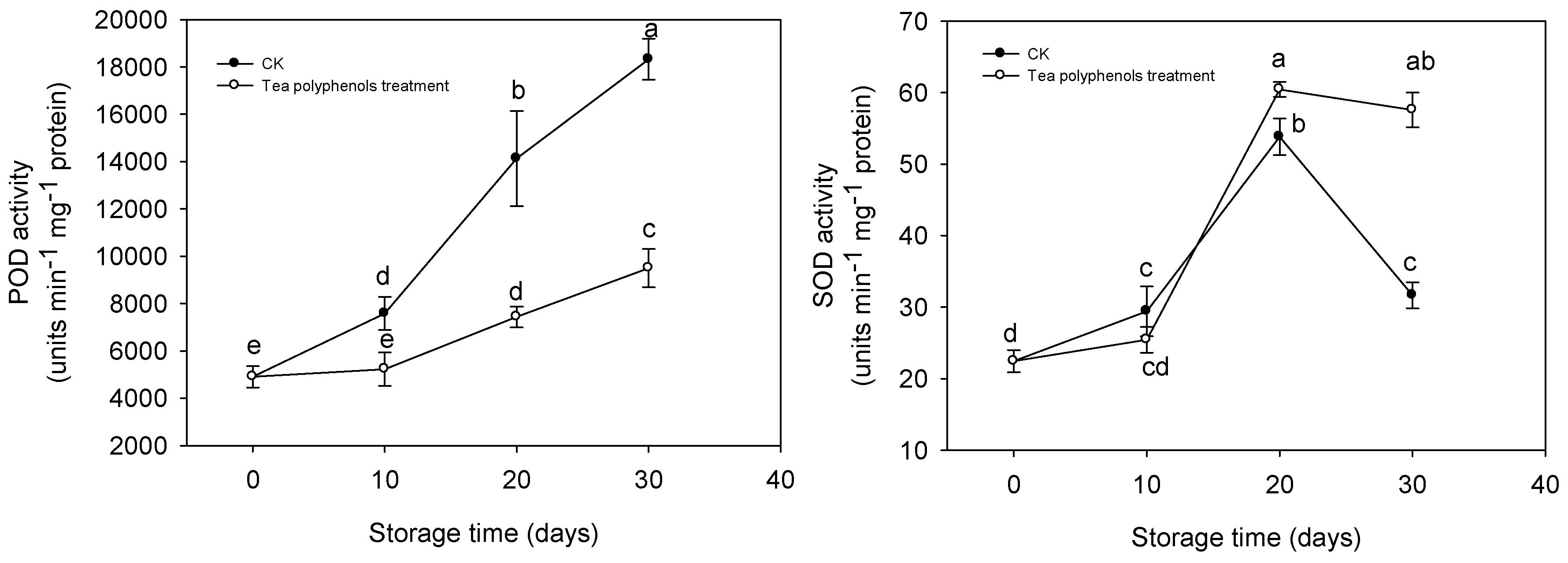
2.7. The Changes in Activities of Antioxidative Enzymes
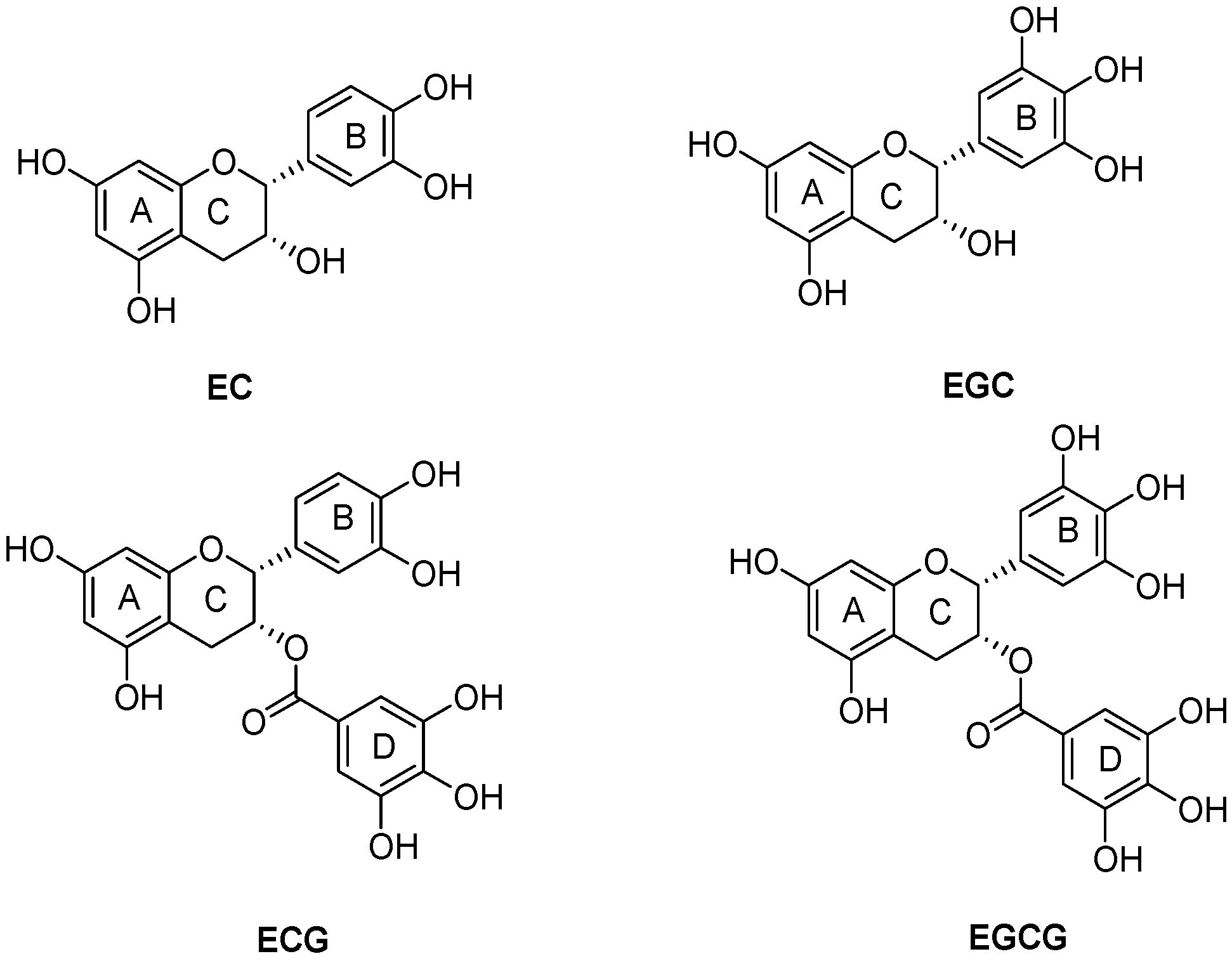
2.8. The Changes in Phenolic Compounds in Pericarp Tissues of Litchi Fruit during Cold Storage

| Phenolic Compounds | Before Storage | Control | Tea Polyphenols |
|---|---|---|---|
| Procyanidin B1 | 0.038 ± 0.004 a | 0.034 ± 0.005 a,b | 0.027 ± 0.001 b |
| Catechin | 0.293 ± 0.008 a | 0.186 ± 0.002 c | 0.253 ± 0.003 b |
| Epicatechin | 2.532 ± 0.108 a | 1.229 ± 0.016 c | 1.913 ± 0.045 b |
| Epicatechin 3-gallate | 1.422 ± 0.062 a | 0.729 ± 0.007 c | 1.188 ± 0.047 b |
3. Discussion
4. Experimental Section
4.1. Plant Materials and Treatments
4.2. Browning Index Assessment
4.3. Determinations of Contents of Ascorbic Acid and Total Soluble Solids
4.4. Analyses of Membrane Permeability and Lipid Peroxidation
4.5. Measurements of H2O2 and O2.− Contents
4.6. Determinations of Activities of Superoxide Dismutase and Peroxidase
4.7. Determination of 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Activity
4.8. Determinations of Contents of Total Phenols and Anthocyanins
4.9. Analyses of Phenolic Compounds
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, Y.M.; Duan, X.W; Joyce, D.; Zhang, Z.Q.; Li, J.R. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar]
- Huang, S.; Hart, H.; Lee, H.; Wicker, L. Enzymatic and color changes during post-harvest storage of lychee fruit. J. Food Sci. 1990, 55, 1762–1763. [Google Scholar] [CrossRef]
- Lin, Z.F.; Li, S.S.; Zhang, D.L.; Liu, S.X.; Li, Y.B.; Lin, G.Z.; Chen, M.D. The changes of oxidation and peroxidation in postharvest litchi fruit. Acta Bot. Sin. 1988, 30, 383–387. [Google Scholar]
- Duan, X.W.; Su, X.G.; Shi, J.; You, Y.L.; Zhao, M.M.; Li, Y.B.; Wang, Y.; Jiang, Y.M. Inhibitory effect of anthocyanin extract from seed coat of black bean on pericarp browning and lipid peroxidation of litchi fruit during storage. J. Food. Biochem. 2008, 32, 415–430. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Yang, B.; Lin, H.T.; Chen, F.; Jiang, Y.M. Degradation of anthocyanin from litchi fruit pericarp by H2O2 and hydroxyl radical. Food Chem. 2009, 116, 995–998. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.W.; Jiang, Y.M.; Su, X.G.; Zhang, Z.Q.; Shi, J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1365–1371. [Google Scholar]
- Jiang, Y.M.; Fu, J.R. Inhibition of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. Food Chem. 1998, 62, 49–52. [Google Scholar] [CrossRef]
- Kanwar, J.; Mujtaba Taskeen, I.M.; Huo, C.D.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, Y.; Song, L.; Liu, H.; Lichter, A.; Kerdchoechuen, O.; Joyce, D.C.; Shi, J. Postharvest characteristics and handling of litchi fruit—An overview. Anim. Prod. Sci. 2006, 46, 1541–1556. [Google Scholar] [CrossRef]
- Pizzocaro, F.; Torreggiani, D.; Gilardi, G. Inhibition of apple polyphenoloxidase (PPO) by ascorbic acid, citric acid and sodium chloride. J. Food Process. Preserv. 1993, 17, 21–30. [Google Scholar] [CrossRef]
- Feng, X.Q.; Biasi, B.; Mitcham, E.J. Effects of various coatings and antioxidants on peel browning of ‘Bartlett’ pears. J. Sci. Food Agric. 2004, 84, 595–600. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Pang, X.Q.; Duan, X.W.; Ji, Z.L.; Jiang, Y.M. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Lamikanra, O.; Watson, M.A. Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J. Food Sci. 2001, 66, 1283–1286. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant enzymes and DPPH-radical scavenging activity in chilled and heat-shocked rice (Oryza sativa L.) seedlings radicles. J. Agric. Food Chem. 2002, 50, 513–518. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; Tanaka, M. Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem. 2005, 90, 231–239. [Google Scholar] [CrossRef]
- Harold, N.G. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Zhao, B.L.; Li, X.J.; He, R.G.; Cheng, S.J.; Xin, W.J. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989, 14, 175–185. [Google Scholar]
- Poei-langston, M.S.; Wrolstad, R.E. Color degradation in an ascorbic acid-anthocyanin-flavanol model system. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Jiang, Y.M. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Fang, C.; Li, Y.B.; Mianda, C. The production of ethylene in litchi fruit during storage and its control. Acta Hortic. Sin. 1986, 13, 151–156. [Google Scholar]
- Jiang, Y.M.; Li, J.R.; Shi, J. Enhanced effect of l-cysteine and citric acid combination on browning inhibition and quality maintenance in harvested litchi fruit. J. Food Sci. Technol. Mysore 2008, 45, 75–77. [Google Scholar]
- Guidi, L.; Tonini, M.; Soldatini, G.F. Effects of high light and ozone fumigation on photosynthesis in Phaseolus vulgaris. Plant Physiol. Biochem. 2000, 38, 717–725. [Google Scholar] [CrossRef]
- Belinky, P.A.; Flikshtein, N.; Dosoretz, C.G. Induction of lignin peroxidase via reactive oxygen species in manganese-deficient cultures of Phanerochaete chrysosporium. Enzym. Microb. Technol. 2006, 39, 222–228. [Google Scholar] [CrossRef]
- Fath, M.A.; Diers, A.R.; Aykin-Burns, N.; Simons, A.L.; Hua, L.; Spitz, D.R. Mitochondrial electron transport chain blockers enhance 2-deoxy-D-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biol. Ther. 2009, 8, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhang, Z.; Xuequn, P.; Yang, C.; Ji, Z.; Jiang, Y. Purification and structural analysis of anthocyanins from litchi pericarp. Food Chem. 2004, 84, 601–604. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C.; Grigor, J.M. Changes in phenolic compounds in Litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Postharvest Biol. Technol. 2000, 19, 165–172. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, Z.; Shen, Y.; Duan, X.; Jiang, Y. Effect of Tea Polyphenols on Lipid Peroxidation and Antioxidant Activity of Litchi (Litchi chinensis Sonn.) Fruit during Cold Storage. Molecules 2014, 19, 16837-16850. https://doi.org/10.3390/molecules191016837
Chen W, Zhang Z, Shen Y, Duan X, Jiang Y. Effect of Tea Polyphenols on Lipid Peroxidation and Antioxidant Activity of Litchi (Litchi chinensis Sonn.) Fruit during Cold Storage. Molecules. 2014; 19(10):16837-16850. https://doi.org/10.3390/molecules191016837
Chicago/Turabian StyleChen, Wenrong, Zhenzhen Zhang, Yanwen Shen, Xuewu Duan, and Yuemin Jiang. 2014. "Effect of Tea Polyphenols on Lipid Peroxidation and Antioxidant Activity of Litchi (Litchi chinensis Sonn.) Fruit during Cold Storage" Molecules 19, no. 10: 16837-16850. https://doi.org/10.3390/molecules191016837





